PO43− polyanion-doping for stabilizing Li-rich layered oxides as cathode materials for advanced lithium-ion batteries†
Abstract
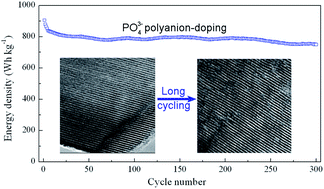
Advanced Li-ion batteries, with Li-rich layered oxides as cathode materials and Si-based composites as anode materials, are considered as high energy battery systems for the next generation of smart communications and electric vehicles (EVs). At the current stage, it is significant to develop Li-rich layered oxides with stable output energy density. However, the gradual capacity degradation and potential decay during cycling lead to the continual decrease in the energy density of Li-rich layered oxides. Therefore, a new strategy should be introduced to block the migration of transition metal cations and maintain the parent layered structure during cycling, in order to stabilize the energy density of oxides. In this work, large tetrahedral PO43− polyanions with high electronegativity with respect to O2− anions are doped into oxides for minimizing the local structure change during cycling. When doping with PO43− polyanions, the parent layered structure is retained during long cycling, due to the strong bonding of PO43− polyanions to transition metal cations (Ni especially). Correspondingly, PO43− polyanion-doped oxides present excellent energy density retention during long cycling, integrated with the discharge capacity and midpoint potential. These results suggest that polyanion-doping can meet the performance requirement of stabilizing the energy density of Li-rich layered oxides for advanced lithium ion batteries.

 Please wait while we load your content...
Please wait while we load your content...