The role of composition in the atomic structure, oxygen loss, and capacity of layered Li–Mn–Ni oxide cathodes†
Abstract
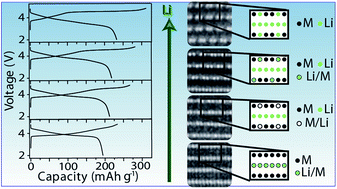
Lithium-rich layered Li[Li1/3−2x/3Mn2/3−x/3Nix]O2 (0 < x ≤ 1/2) oxide cathodes show promise as a potential candidate for Li-ion batteries due to their high capacity. However, the intricacies of the role of composition with increasing excess Li on the degree of oxygen loss during the first charge and the discharge capacity in subsequent cycles are not fully understood. With an aim to develop a better fundamental understanding, we present here an in-depth investigation of the Li[Li1/3−2x/3Mn2/3−x/3Nix]O2 (0 < x ≤ 1/2) series with a range of different excess lithium contents prepared by two different synthesis methods. The oxygen loss from the lattice during the first charge and the discharge capacity in subsequent cycles increase with increasing lithium content. In-depth analysis with a combination of X-ray diffraction, scanning electron microscopy (SEM), aberration-corrected scanning transmission electron microscopy (STEM), diffraction-STEM (D-STEM), and energy dispersive X-ray spectroscopy (EDS) reveals that the samples transition from an R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m structure to a C2/m structure with increasing lithium content and decreasing nickel to manganese ratio, for both the synthesis methods, indicating that the maximum oxygen loss and discharge capacity are achieved with a single C2/m phase. We further show that within a single particle, the cation layers of these materials can order on different {111} planes in the basic NaCl structure.
m structure to a C2/m structure with increasing lithium content and decreasing nickel to manganese ratio, for both the synthesis methods, indicating that the maximum oxygen loss and discharge capacity are achieved with a single C2/m phase. We further show that within a single particle, the cation layers of these materials can order on different {111} planes in the basic NaCl structure.

 Please wait while we load your content...
Please wait while we load your content...