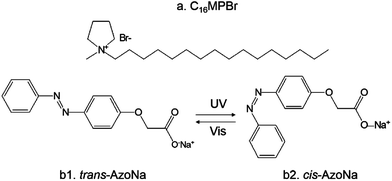
Photo-induced transformation from wormlike to spherical micelles based on pyrrolidinium ionic liquids†
Han
Yan
ab,
Yue
Long
b,
Kai
Song
b,
Chen-Ho
Tung
b and
Liqiang
Zheng
*a
aKey Laboratory of Colloid and Interface Chemistry, Shandong University, Ministry of Education, Jinan, Shandong 250100, P.R. China. E-mail: lqzheng@sdu.edu.cn; Fax: +86-531-88564750; Tel: +86-531-88366062
bBeijing National Laboratory for Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing, 100190, P.R. China
First published on 16th October 2013
Abstract
A UV-induced self-assembly based on N-methyl-N-cetylpyrrolidinium bromide (C16MPBr) and sodium (4-phenylazo-phenoxy)-acetate (AzoNa) was fabricated in the present work through facile mixing of C16MPBr and trans-AzoNa in water. Due to the trans–cis photoisomerization functionality of the AzoNa, this self-assembly possesses photo-responsive properties. Under ambient conditions, the morphology of the self-assembly was wormlike micelles with a high viscosity of about 10 Pa s, whereas after applying UV light, the self-assembly transformed into spherical micelles with a dramatically lowered viscosity of about 0.01 Pa s. Different from most reported photo-responsive wormlike micelles consisting of conventional cationic surfactant CTAB, our system was constructed from a novel pyrrolidinium ionic liquid. We hope this study may provide a deeper understanding of photo-responsive wormlike micelles constructed by cationic surfactants and photo-sensitive additives in water.
Introduction
When solubilized in aqueous solutions, surfactants, which have both hydrophobic and hydrophilic moieties on the same molecule, can form various highly organized structures in a micrometer or even nanometer length scale, such as micelles,1 vesicles,2 fibers,3 and lamellar phases,4etc. As one of the most important self-assemblies, long and flexible wormlike micelles have received considerable attention over the past few decades.5–10 Above a system dependent concentration of the surfactant, which is called the overlapping concentration (c*), wormlike micelles may entangle into a transient network, and exhibit remarkable viscoelastic properties like a flexible polymer solution.11,12 However, unlike polymers, the network constantly breaks and recombines,13 imparting complex rheological response to the solution. Because of their elongated, cylindrical shape and distinct rheological behaviour, wormlike micelles have been applied in numerous fields such as rheology control,14 cosmetic products,15 and enhanced oil recovery.16Recently, smart wormlike micelles,17 whose physicochemical properties can be instantaneously induced by external stimuli, have been intensively reported. Chu et al. summarized types of stimuli to manipulate the response of smart wormlike micelles and discussed the potential applications and future challenges of smart wormlike micelles. According to their review, these stimuli are generally summarized as several classifications: redox reaction,18 UV/Vis light,19 temperature,20 pH,21 CO2,22 hydrocarbon23 and combined stimuli.24 Among these environmental stimuli, the UV/Vis trigger is considered to be of great importance and superiority,17,25,26 and has been widely applied. Firstly, compared to pH changes, redox reagent, stress, or salinity, light is much gentle and non-invasive, since no additives are needed. Secondly, as a kind of mild energy source, light signal is much convenient to get and is the most reliable strategy to modulate molecular assembly. Thirdly, unlike electric and sound waves, light can be located at a precise spatial place, which is of special value in nanoscience and medical applications.
During the past decade, many scientists have dedicated themselves to the investigation of light-induced self-assembling behaviour in aqueous solutions. Huang et al. used AzoNa as a binary-state molecular switch to create photo-modulated multi-state and multi-scale molecular assemblies in cetyltrimethylammonium bromide (CTAB) solutions. The molecular assemblies including wormlike micelles, vesicles, planar lamellae, cylindrical and spherical micelles can be reversibly controlled by exposing to UV or visible light.25 Wolf and coworkers found that wormlike micelles can be formed in a CTAB solution in the presence of a series of 9-alkyl-substituted nonpolar anthracene derivatives.27 Abe et al. utilized the reversible trans–cis photoisomerization of an azobenzene modified cationic surfactant as a photo-switch to control changes in fluid viscosity.28 Raghavan et al. reported a new class of photo-rheological fluids that undergoes a transition from wormlike to short rodlike micelles.29 Zhang and coworkers have achieved regulation of the morphology of aggregations via UV and visible light using a photo-responsive malachite green molecule (MGCB) and pyrene with a surfactant (PYN).30 Wang et al. studied the phase behaviour of a tetradecyldimethylamine oxide (C14DMAO) and para-coumaric acid (PCA) solution, and observed a novel phase transition induced by UV irradiation.26
Surface active ILs, which have a hydrophilic headgroup and a hydrophobic tail analogous to traditional ionic surfactants, have received great attention in recent years.31 Due to their inherent amphiphilic properties, they can be self-assembled into a variety of aggregates in aqueous solutions, such as microemulsions,32 hydrogels,33 vesicles,34 and wormlike micelles.35 We have investigated the vesicles formed by 1-dodecyl-3-methylimidazolium bromide (C12mimBr) and sodium dodecyl sulfate (SDS) in water.34 Huang et al. reported pH- and therm-responsive hydrogels consisting of C16mimBr and salicylate sodium (NaSal).36 However, studies related to the self-assemblies formed by surface active ILs have mostly focused on imidazolium ILs, but rarely involved ILs consisting of different cationic headgroups.37
In this paper, a novel complex fluid was facilely constructed by mixing a pyrrolidinium IL, N-methyl-N-cetylpyrrolidinium bromide (C16MPBr), and a photosensitive azobenzene-containing molecule in water. After exposing to UV for a certain time, the self-assembled morphology transformed from long and flexible wormlike micelles to spherical micelles. The UV-triggered trans–cis isomerization of photosensitive sodium (4-phenylazo-phenoxy)-acetate (AzoNa) is of great importance in this system. Under UV irradiation, trans-AzoNa converted to its cis form, which could deeply influence the hydrophobic–hydrophilic balance of the molecule, thus affecting the structure of the aggregates. Different from Huang's report25 based on CTAB solutions, our photo-responsive self-assemblies were constructed by using a novel pyrrolidinium type surface active IL. We expect this paper to widen the types of surfactants which could be stimulated by external environment into self-assembled aggregates.
Experimental
Materials
C16MPBr (Scheme 1a) and sodium (4-phenylazo-phenoxy)-acetate (AzoNa) (Scheme 1b) were synthesized and purified using the methods reported previously.25 The water was triply distilled. Solutions of C16MPBr were prepared by directly dissolving the solid IL-type surfactant in water, and a desired amount (10–80 mM) of AzoNa was added to the resulting solution. As AzoNa has a very low solubility in water at room temperature, the samples were kept for heating until AzoNa was totally dissolved. Homogeneous solutions with an orange color were obtained after heating and stirring for several minutes. The solutions were then left to reach the equilibrium at 25 °C for several days before the measurements. All the measurements were conducted in a neutral environment.Characterization
Results and discussion
Photo-induced trans–cis transition of AzoNa
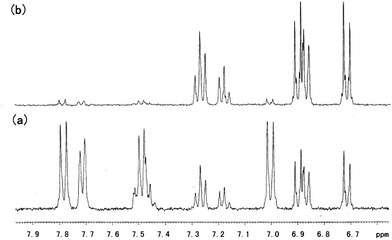
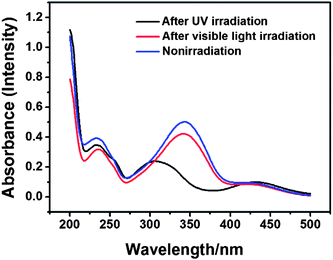
AzoNa, which contains an azobenzene group, was synthesized according to Huang's report.25 Under UV irradiation, the molecular structure of AzoNa could undergo a transition from trans (Scheme 1b1) to cis (Scheme 1b2) state. As calculated from the 1H NMR spectrum of aromatic protons in this azobenzene derivative molecule, there are about 67% trans-AzoNa and 34% cis-AzoNa before UV irradiation (Fig. 1a). When the sample was exposed to UV-light for 30 min, the percentage of trans-AzoNa drastically reduced to 5%, while the cis form increased to 95% (Fig. 1b). UV irradiation also caused a further upfield shift of aromatic protons in the cis-AzoNa than the trans-AzoNa, which could be explained by the magnetically anisotropic effect.The absorbance spectra of the AzoNa were obtained to further confirm the trans–cis transition. Fig. 2 shows the absorbance spectra of an aqueous AzoNa solution at different UV irradiation times. Before light irradiation, an absorption band ascribed to the π–π* transition of trans-AzoNa was observed at 344 nm.25 UV irradiation on the solution resulted in the remarkable decrease in intensity and blue-shift of this absorption peak. The changes of the absorption band in the spectra confirmed the photoisomerization of trans-AzoNa to cis-AzoNa. It also can be found that within 30 min, a photostationary state was attained. The photo-induced trans–cis transition of AzoNa was also reversible, as shown in Fig. 3. If the UV-irradiated solution was subsequently irradiated by visible light for about 24 hours, the absorption of the cis-isomer decreased while the absorption band at 344 nm which was assigned to trans-AzoNa appeared. This result proves the reversible trans–cis photoisomerization of AzoNa. However, it should be noted that, in Fig. 3, the intensity of the absorption peak at 344 nm after visible light irradiation was slightly lower compared to the primary state before UV and visible light irradiation, indicating that the transition of cis-AzoNa back to its trans-isomer was not complete yet.38
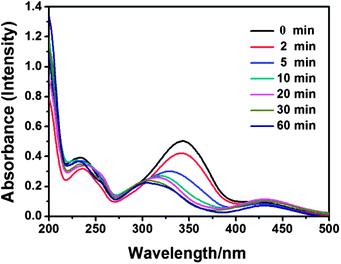 | ||
| Fig. 2 UV-vis absorption spectra of an aqueous AzoNa solution (50 mM) at different times of UV-light irradiation. | ||
Photo-switched molecular self-assemblies based on AzoNa and C16MPBr
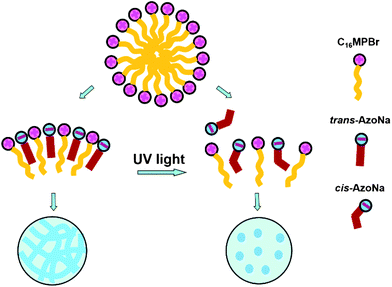
Because of its special trans–cis isomerization under the irradiation of UV-light, AzoNa can be regarded as a dual-state molecular switch. When a certain amount of AzoNa was added to a 100 mM C16MPBr aqueous solution, a highly viscoelastic gel-like solution was formed. However, after UV irradiation for 30 min, the sample changed into a runny fluid with much lower viscosity. This drastic decrease in viscosity may be attributed to the change of the aggregation state of molecules in the sample. The reversible trans to cis photoisomerization of the double bond in AzoNa altered its molecular geometry, which directly influenced the packing behaviour of C16MPBr and AzoNa molecules in aqueous solution. As a consequence, the sample experienced a transition from long, flexible wormlike micelles into spherical micelles with the extension of the UV irradiation time (Scheme 2). In order to confirm the above hypothesis, rheological measurements were conducted on the sample.
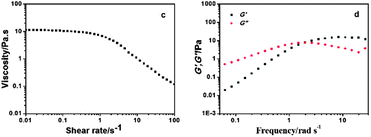
Fig. 4 displays typical rheological results of the sample consisting of 100 mM C16MPBr and 50 mM AzoNa. As the steady shear rheological curve in Fig. 4a shows, before UV irradiation, the sample exhibited shear-thinning behaviour with a Newtonian plateau of about 10 Pa s (about 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times larger than that of pure water) at lower shear rates; while at higher shear rates, a notable decrease in viscosity can be clearly observed, which was caused by the alignment of the wormlike chain under the direction of flow.20 Additionally, dynamic rheological behaviour, considered as a very sensitive nanostructure probe in complex fluids, was further examined. As shown in Fig. 4b, at lower frequency, both the elastic modulus G′ and the viscous modulus G′′ increased with the increase of the frequency, and G′′ was slightly greater than G′; However, at higher frequency, G′ was dominant with a plateau value (G0), whereas G′′ first decreased and then increased. It is confirmed that similar rheological behaviours also exist in samples containing 100 mM C16MPBr and 40, 60, 70 and 80 mM AzoNa (see the ESI†). This phenomenon is quite accordant with Maxwell's mechanical model, which can be used to describe the dynamic rheological behaviours of a viscoelastic micellar solution. The model can be expressed as follows:
000 times larger than that of pure water) at lower shear rates; while at higher shear rates, a notable decrease in viscosity can be clearly observed, which was caused by the alignment of the wormlike chain under the direction of flow.20 Additionally, dynamic rheological behaviour, considered as a very sensitive nanostructure probe in complex fluids, was further examined. As shown in Fig. 4b, at lower frequency, both the elastic modulus G′ and the viscous modulus G′′ increased with the increase of the frequency, and G′′ was slightly greater than G′; However, at higher frequency, G′ was dominant with a plateau value (G0), whereas G′′ first decreased and then increased. It is confirmed that similar rheological behaviours also exist in samples containing 100 mM C16MPBr and 40, 60, 70 and 80 mM AzoNa (see the ESI†). This phenomenon is quite accordant with Maxwell's mechanical model, which can be used to describe the dynamic rheological behaviours of a viscoelastic micellar solution. The model can be expressed as follows:
| G′(ω) = G0ω2tR2/(1 + ω2tR2) | (1) |
| G′′(ω) = G0ωtR/(1 + ω2tR2) | (2) |
| tR = 1/ω* | (3) |
| G0 = 2G* | (4) |
Here, ω, G0, and tR are the angular frequency, the plateau modulus and the relaxation time, respectively; whereas ω* and G* represent the critical angular frequency and modulus where the curve of G′ and G′′ intersects.
To easily visualize how well a Maxwell model fits the data, Cole–Cole plots (plots of G′ as a function of G′′) are commonly used. According to the following equation (eqn (5)), Cole–Cole plots of several samples are studied (Fig. 5).
| G′′2 + (G′ − G0/2)2 = (G0/2)2 | (5) |
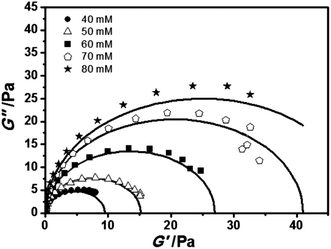 | ||
| Fig. 5 Cole–Cole plots for samples with different AzoNa concentrations at a fixed C16MPBr concentration of 100 mM. The concentrations of AzoNa were 40, 50, 60, 70 and 80 mM, respectively. | ||
As shown in Fig. 5, all the low frequency data correspond to the Maxwell behaviour well, whereas the high frequency data slightly deviate from the semicircle Cole–Cole plots, which might be attributed to the Rouse relaxation modes.13
Based on the above rheological results with visual observations, it can be concluded that long, flexible wormlike micelles were formed in the C16MPBr–AzoNa aqueous solution before UV irradiation. These wormlike micelles may intertwine with each other and form a transient network, endowing the system with highly viscoelastic properties.
When UV irradiation was applied, interesting phenomena were observed. It was found that viscosity of the samples gradually decreased with the increase of the irradiation time. After being irradiated for about 30 min, these samples eventually changed from viscoelastic fluids to flowing fluids with very low viscosity. Fig. 6 shows typical dynamic rheograms of the 100 mM C16MPBr and 50 mM AzoNa system, plotting the elastic modulus G′ and the viscous modulus G′′ as a function of the sweeping frequency before (a) and after (b) UV irradiation. It can be found that without irradiation, the system displayed a representative viscoelastic behaviour: G′′ > G′ in the low frequency region, while G′ > G′′ in the high frequency region. However, after UV irradiation for 30 min, both G′ and G′′ show power law dependence on frequency at whole sweeping scales, which is the typical terminal rheological behaviour of viscous fluids.
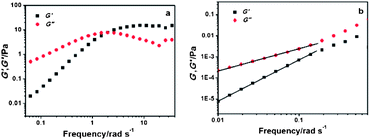 | ||
| Fig. 6 Dynamic frequency sweep of a 100 mM C16MPBr solution with 50 mM AzoNa before (a) and after (b) UV irradiation. | ||
In order to further examine the aggregation behaviour before and after UV irradiation, the cryo-TEM technique was employed. As shown in Fig. 7a, a typical wormlike micellar network of 50 mM trans-AzoNa and 100 mM C16MPBr before irradiation is clearly observed. This three-dimensional network is formed by the intertwining of many wormlike micelles. Fig. 7b presents a magnified picture of these wormlike micelles: with a length of about 600 nm and a diameter less than 10 nm. After irradiation by UV light, however, both length and diameter of the wormlike micelles decrease significantly, and most of the aggregates transform into spherical micelles, as shown in Fig. 7c and d. The size of these spheres decreases to the magnitude of about 10 nm, which is much shorter than that of wormlike micelles. This cryo-TEM result is in good agreement with the rheological result discussed before, which also demonstrates a transition from wormlike to spherical micelles induced by UV irradiation.
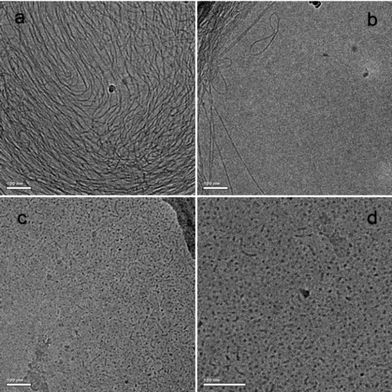 | ||
| Fig. 7 Cryo-TEM image of a 100 mM C16MPBr and 50 mM AzoNa solution before (a and b) and after (c and d) UV irradiation. All the scale bars are 100 nm. | ||
According to the rheology and cryo-TEM experiments, it is the long and flexible wormlike micelles that are responsible for the viscoelastic properties of the sample before irradiation. Combined with the results of rheology and cryo-TEM, we speculate that a light-induced transition from wormlike micelles into spherical micelles occurs in the 100 mM C16MPBr and 50 mM AzoNa system. Unlike wormlike micelles, the shortened micelles existing after irradiation lack sufficient length and flexibility; thus, they cannot entangle with each other to form a transient network. As a result, the sample loses its viscoelasticity and behaves like a less viscous fluid.
In order to demonstrate the influence of UV irradiation on the samples, the zero-shear viscosity η0 before and after irradiation of 100 mM C16MPBr solutions was obtained and plotted as a function of AzoNa concentration (Fig. 8). It can be seen that before irradiation, the viscosity increased until it reached the highest value (∼10 Pa s) at an AzoNa concentration of 50 mM, and then decreased with the addition of AzoNa. A similar phenomenon is also seen in the previous reports of wormlike micelle systems,15,35 where the decrease of viscosity is caused by formation of the branched micelles.7,15 When UV irradiation was applied, a sharp decrease of viscosity could be clearly observed for all the tested samples (i.e., 100 mM C16MPBr and 10–80 mM AzoNa). However, both the initial viscosity value and the extent of viscosity decrease were different for each individual sample. After UV irradation for 30 min, the viscosity of all the samples achieved the minimum, with the biggest diminution of about 600 times. Under such conditions, even the highest viscosity was at 10−2 orders of magnitude, which is much closer to that of pure water.
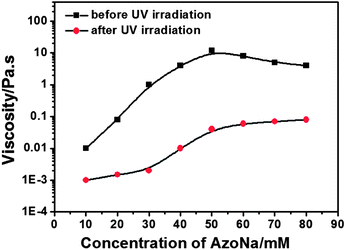 | ||
| Fig. 8 Before and after UV irradiation, variation of the zero-shear viscosity η0 as a function of AzoNa concentration at a fixed 100 mM C16MPBr concentration. | ||
In addition, according to Fig. 4 and S1,† wormlike micelles can be formed in 100 mM C16MPBr and 40–80 mM AzoNa systems. As the molar ratio of C16MPBr/AzoNa was <1, these wormlike micelles should be positively charged.25 The electrostatic attraction between the headgroup of C16MPBr and the [Azo]− as well as cation–π interactions39,40 between the headgroup of C16MPBr and the benzene rings of the AzoNa molecule benefited the formation of wormlike micelles, which endowed the samples with viscoelastic properties. Meanwhile, cation–π interactions should have been strongest when the concentration of AzoNa was increased to 50 mM as it had two benzene rings; so there was a viscosity peak appearing at the molar ratio of C16MPBr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AzoNa equal to 2
AzoNa equal to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
Mechanism of the photo-induced self-assembly process
It is acknowledged that without AzoNa, short and rigid spherical micelles are the dominant self-assembled structures of C16MPBr, with the positively charged pyrrole rings packing at the micellar interface. Due to the electrostatic repulsion between their headgroups, C16MPBr cannot pack closely with each other. At the same time, the effective area per molecule at the surfactant–water interface is relatively large, which is unfavorable for micellar growth. When AzoNa was added to the solution, it was immediately adsorbed to the positively charged headgroup of C16MPBr. The adsorption of AzoNa not only reduced the electrostatic repulsion but also decreased the effective area per molecule packing at the micellar interface.41 As a result, the micelles underwent enormous elongation and finally formed wormlike micelles. Apparently, whether or not AzoNa counterions can tightly associate with the headgroups at the interface comes up to the key point of wormlike micelle formation.Furthermore, it has been proven that for self-assemblies formed by oppositely charged surfactants, there are two major factors that determine whether they can regularly pack and tightly associate: geometry of the two molecules and their hydrophobicities.42 In the present work, the light-induced isomerization of trans/cis AzoNa directly leads to significant changes in their molecular structures, which would further influence the packing and association of C16MPBr and AzoNa. A specific explanation is as follows: as a kind of aromatic counterions, [Azo]− generally absorbs at the interface with its two aromatic rings embedded in the hydrophobic inner core of the micelle, while the more hydrophilic carboxyl group is located close to the pyrrole ring and faces outward the interface. Such an arrangement is obviously more favorable for trans-AzoNa than cis-AzoNa because in trans-AzoNa, the two aromatic rings distribute on different sides of the double bond, while in cis-AzoNa, they both distribute on the same side. Therefore, the trans isomer owns more linear molecular architecture and much lower steric hindrance, making it more easy to penetrate into the micellar interior than the cis isomer. Moreover, it has been reported that trans-AzoNa has a greater hydrophobicity than cis-AzoNa,25,26 demonstrated in their solubility of 25 and 100 mM, respectively. In fact, for many kinds of azobenzene derivatives, the trans isomers are always more hydrophobic, and the cis ones are much more hydrophilic.43 For example, in azobenzene-based surfactants, the more hydrophobic trans form owns a lower critical micelle concentration. We believe that their different hydrophobicities are caused by light-triggered variation of net dipole moment.28,43 A lower dipole moment of the trans isomer gives it a greater hydrophobicity, while a higher dipole moment gives the cis isomer a greater hydrophilicity. As a consequence, trans-AzoNa can effectively penetrate into the interior of the micelles and tightly associate with C16MPBr, while cis-AzoNa cannot.
Based on the discussion above, we finally propose a rational mechanism to our experiments. Without UV-irradiation, trans-AzoNa is the major existing form of AzoNa. When mixed with C16MPBr, a relatively lower steric hindrance and a greater hydrophobicity enabled it to interact strongly with C16MPBr and favored the micellar growth to wormlike micelles. Under UV-irradiation, the trans isomer gradually converted into its cis form, accompanied by an increase in steric hindrance and a decrease in hydrophobicity. Therefore, when cis-AzoNa became the majority, the combination of AzoNa and C16MPBr was greatly weakened and AzoNa was partially desorbed from the micelles, resulting in an increase in the effective area of hydrophilic headgroups, which was unfavorable for micellar growth. As a result, the micelles were greatly shortened in length and could not entangle with each other, resulting in a sharp drop of the viscosity value.
Conclusions
In conclusion, we have designed a novel photo-induced self-assembly system based on long chain pyrrolidinium ILs and a photo-sensitive azobenzene-containing molecule. Absorbance spectroscopy, NMR, cryo-TEM and rheological measurements were utilized and proved the self-assembly transition from long and flexible wormlike micelles to short and rigid spherical micelles induced by UV irradiation. The molecule structure variation of trans- and cis-AzoNa induced by UV-light played a vital role in this transition, and it is the first time that pyrrolidinium type surface active ILs have been used to construct a photo-responsive self-assembly system. It is expected that this work may help to provide a deeper understanding of the phase behaviours of pyrrolidinium ILs and to achieve further applications of wormlike micelles.Acknowledgements
The authors are grateful to the National Basic Research Program (2013CB834505), the National Natural Science Foundation of China (no. 91127017), and the Specialized Research Fund for the Doctoral Program of Higher Education of China (no. 20120131130003). We also would like to thank the Center for Biological Imaging (CBI), Institute of Biophysics, Chinese Academy of Science for our cryo-TEM work and we are grateful to Gang Ji for his help in making samples and taking images.Notes and references
- Y. Y. Won, H. T. Davis and F. S. Bates, Science, 1999, 283, 960 CrossRef CAS.
- T. Kunitake and Y. Okahata, J. Am. Chem. Soc., 1977, 99, 3860 CrossRef CAS.
- H. W. Jun, S. E. Paramonov and J. D. Hartgerink, Soft Matter, 2006, 2, 177 RSC.
- J. C. Hao and H. Hoffman, Curr. Opin. Colloid Interface Sci., 2004, 9, 279 CrossRef CAS PubMed.
- R. Zana and Y. Talmon, Nature, 1993, 362, 228 CrossRef CAS.
- D. Danino, Y. Talmon, H. Levy, G. Beinert and R. Zana, Science, 1995, 269, 1420 CrossRef CAS PubMed.
- S. R. Raghavan and E. W. Kaler, Langmuir, 2001, 17, 300 CrossRef CAS.
- V. Croce, T. Cosgrove, C. A. Dreiss, S. King, G. Maitland and T. Hughes, Langmuir, 2005, 21, 6762 CrossRef CAS PubMed.
- Y. Han, Y. Feng, H. Sun, Z. Li, Y. Han and H. Wang, J. Phys. Chem. B, 2011, 115, 6893 CrossRef CAS PubMed.
- H. Afifi, G. Karlsson, R. K. Heenan and C. A. Dreiss, Langmuir, 2011, 27, 7480 CrossRef CAS PubMed.
- M. E. Cates, Macromolecules, 1987, 20, 2289 CrossRef CAS.
- H. Rehage and H. Hoffmann, Mol. Phys., 1991, 74, 933 CrossRef CAS.
- D. P. Acharya and H. Kunieda, Adv. Colloid Interface Sci., 2006, 123–126, 101 Search PubMed.
- M. R. Stukan, E. S. Boek, J. T. Padding, W. J. Briels and J. P. Crawshaw, Soft Matter, 2008, 4, 870 RSC.
- J. Yang, Curr. Opin. Colloid Interface Sci., 2002, 7, 276 CrossRef CAS.
- C. Watkins, Inform, 2009, 20, 682 Search PubMed.
- Z. Chu, C. A. Dreiss and Y. Feng, Chem. Soc. Rev., 2013, 42, 7174 RSC.
- K. Tsuchiya, Y. Orihara, Y. Kondo, N. Yoshino, T. Ohkubo, H. Sakai and M. Abe, J. Am. Chem. Soc., 2004, 126, 12282 CrossRef CAS PubMed.
- J. Li, M. W. Zhao, H. T. Zhou, H. J. Gao and L. Q. Zheng, Soft Matter, 2012, 8, 7858 RSC.
- Z. Chu and Y. Feng, Chem. Commun., 2011, 47, 7191 RSC.
- Z. Chu and Y. Feng, Chem. Commun., 2010, 46, 9028 RSC.
- Y. Lin, P. G. Jessop, M. Cunningham, C. A. Eckert and C. L. Liotta, Science, 2006, 313, 958 CrossRef PubMed.
- H. Hoffmann and G. Ebert, Angew. Chem. Int. Ed. Engl., 1988, 27, 902 CrossRef.
- L. Jiang, K. Wang, F. Ke, D. Liang and J. Huang, Soft Matter, 2009, 5, 599 RSC.
- Y. Lin, X. Cheng, Y. Qian, C. Yu, Z. Li, Y. Yan and J. Huang, Soft Matter, 2010, 6, 902 RSC.
- D. Wang, R. Dong, P. Long and J. Hao, Soft Matter, 2011, 7, 10713 RSC.
- N. Müller, T. Wolff and G. V. Bünau, J. Photochem., 1984, 24, 37 CrossRef.
- H. Sakai, Y. Orihara, H. Kodashima, A. Matsumura, T. Ohkubo, K. Tsuchiya and M. Abe, J. Am. Chem. Soc., 2005, 127, 13454 CrossRef CAS PubMed.
- A. M. Ketner, R. Kumar, T. S. Davies, P. W. Elder and R. S. Raghavan, J. Am. Chem. Soc., 2007, 129, 1553 CrossRef CAS PubMed.
- C. Wang, Q. Chen, H. Xu, Z. Wang and X. Zhang, Adv. Mater., 2010, 22, 2553 CrossRef CAS PubMed.
- B. Chamiot, C. Rizzi, L. Gaillon, J. Sirieix-Plenet and J. Lelievre, Langmuir, 2009, 25, 1311 CrossRef CAS PubMed.
- X. W. Li, J. Zhang, L. Q. Zheng, B. Chen, L. Z. Wu, F. F. Lv, B. Dong and C. H. Tung, Langmuir, 2009, 25, 5484 CrossRef CAS PubMed.
- Y. R. Zhao, X. Chen, B. Jing, X. D. Wang and F. M. Ma, J. Phys. Chem. B, 2008, 113, 983 CrossRef PubMed.
- J. Yuan, X. T. Bai, M. W. Zhao and L. Q. Zheng, Langmuir, 2010, 26, 11726 CrossRef CAS PubMed.
- B. Dong, J. Zhang, L. Q. Zheng, S. Q. Wang, X. W. Li and T. Inoue, J. Colloid Interface Sci., 2008, 319, 338 CrossRef CAS PubMed.
- Y. Lin, Y. Qiao, Y. Yan and J. Huang, Soft Matter, 2009, 5, 3047 RSC.
- H. Yan, M. W. Zhao and L. Q. Zheng, Colloids Surf., A, 2011, 392, 205 CrossRef CAS PubMed.
- X. Tong, G. Wang, A. Soldera and Y. Zhao, J. Phys. Chem. B, 2005, 109, 20281 CrossRef CAS PubMed.
- D. A. Dougherty, Science, 1996, 271, 163 CAS.
- B. D. Frounfelker, G. C. Kalur, B. H. Cipriano, D. Danino and S. R. Raghavan, Langmuir, 2009, 25, 167 CrossRef CAS PubMed.
- M. Vermathen, P. Stiles, S. J. Bachofer and U. Simonis, Langmuir, 2002, 18, 1030 CrossRef CAS.
- A. M. Ketner, R. Kumar, T. S. Davies, P. W. Elder and S. R. Raghavan, J. Am. Chem. Soc., 2007, 129, 1553 CrossRef CAS PubMed.
- C. T. Lee, K. A. Smith and T. A. Hatton, Macromolecules, 2004, 37, 5397 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Rheological results of samples containing 100 mM C16MPBr and 40, 60, 70 and 80 mM AzoNa. See DOI: 10.1039/c3sm52346b |
| This journal is © The Royal Society of Chemistry 2014 |