Facile one-step solid-phase synthesis of multitopic organic–DNA hybrids via “click” chemistry†
Abstract
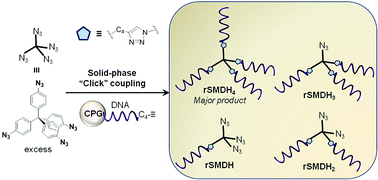
A broad range of synthetically challenging-to-access small molecule–DNA hybrids can be readily synthesized in “one pot” and in high yields by coupling multi-azide cores to alkyne-modified DNAs on a solid support using click chemistry. The multi-functional products can be obtained in pure forms and on large scales (1 μmol) in a facile fashion. In addition, the distribution of the products can be controlled by changing the concentration of the azide core in solution and the strand-loading density on the solid-supports.

 Please wait while we load your content...
Please wait while we load your content...