Iodoarene-catalyzed fluorination and aminofluorination by an Ar-I/HF·pyridine/mCPBA system†
Abstract
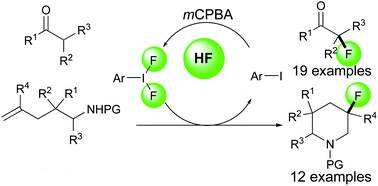
We have developed the iodoarene-catalyzed nucleophilic fluorination of β-dicarbonyl compounds and intramolecular aminofluorination of ω-amino-alkenes using the same reaction conditions. The key for this reaction is the in situ generation of a hypervalent iodine compound ArIF2 by hydrogen fluoride, mCPBA and a catalytic amount of iodoarene. Preliminary trials of catalytic asymmetric nucleophilic fluorination were conducted.

 Please wait while we load your content...
Please wait while we load your content...