Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Lipophilic balance – a new design principle for transmembrane anion carriers†
Hennie
Valkenier
a,
Cally J. E.
Haynes
b,
Julie
Herniman
b,
Philip A.
Gale
*b and
Anthony P.
Davis
*a
aSchool of Chemistry, University of Bristol, Cantock's Close, Bristol, BS8 1TS, UK. E-mail: anthony.davis@bristol.ac.uk
bChemistry, University of Southampton, Southampton, SO17 1BJ, UK. E-mail: philip.gale@soton.ac.uk
First published on 10th January 2014
Abstract
Despite extensive interest in transmembrane anion carriers (anionophores), the factors that govern activity are still only partly understood. Herein we report a study which identifies a new principle for anionophore design, that of “lipophilic balance”. A series of simple thioureas with identical molecular formulae has been prepared and assayed for chloride/nitrate transport activity in synthetic vesicles. The molecules differ only in the positioning of the phenylthiourea binding unit within an 11-carbon linear chain. They are shown to possess very similar lipophilicities and anion affinities, while a test for leaching establishes that they locate almost exclusively in the vesicle membranes. Notwithstanding their close similarities, activities across the series show >5-fold variation, peaking when the phenylthiourea group is centrally located. The results suggest that transport is favoured by a balanced array of lipophilic substituents, possibly because this arrangement facilitates transfer of the complexed anion into the apolar membrane interior.
Introduction
The design of molecules which can transport anions across phospholipid membranes has become a major objective in supramolecular chemistry.1 Interest has been generated by several factors. Firstly there is a natural desire to balance the long-established cation-transporting activity of crown ethers etc. with anion-transporting counterparts. Secondly, there is curiosity regarding the disparity between cation- and anion-transporting natural products. While the former constitute a major family of secondary metabolites (examples being valinomycin, monensin, lasalocid A and the gramicidins),2 there are very few examples of the latter.1a Thirdly, and consequently, there is a need for anion transporters to serve as research tools for membrane biophysics, complementing the roles played by cationophores (notably valinomycin). Fourthly, and perhaps most importantly, there is the chance of discovering new modes of biological activity leading to medical applications. In particular, there are several genetic conditions which are caused by defects in endogenous anion channels, notable examples being Best disease, Bartter's syndrome and cystic fibrosis.3 In principle, anion transporters might be used to treat these conditions by replacing the missing activity, in an approach termed “channel replacement therapy”.4Anion transport may be achieved by a number of strategies including self-assembled channels,5 relay systems,6 and mobile carriers.7–24 For biological applications, carriers are probably favoured as they do not require cooperative action by several molecules and hence can function at low dose. Carriers may be positively charged, forming neutral complexes which can easily pass through membrane interiors, or may acquire positive charge through protonation. However lipophilic cations tend to be toxic, and protonatable molecules may also be capable of proton transport. Thus, for channel replacement therapy, the most promising approach is arguably the use of electroneutral anion carriers. Such molecules mirror the action of valinomycin, the well-studied electroneutral cation carrier, and may thus be termed “anti-valinomycins”.1f
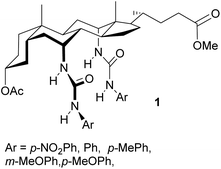
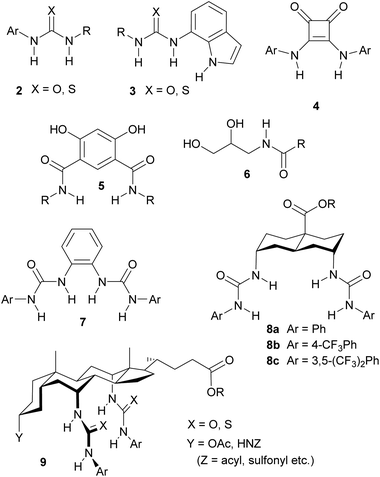
To serve as an anti-valinomycin, a molecule must be able to bind the target anion from water, without involving a counter-cation, and ferry it through the membrane interior. The feasibility of this process was first demonstrated in 2003, when the “cholapod” bis-ureas 1 were shown to exchange chloride and nitrate across synthetic vesicle and natural cell membranes.7 Further work has shown that a variety of neutral molecules, some far less sophisticated than 1, can act as anion carriers. As illustrated in Fig. 1, examples include simple mono-ureas and -thioureas 2,8–11 (thio)ureidoindoles 3,9,10,15 squaramides 4,16 isophthalamides 5,17,18 hydroxyamides 6,19 diureido-benzenes 720 and -decalins 8,21 and a variety of cholapods 9.7,8,22 A number of principles governing anionophore effectiveness have emerged from this research. Firstly there is a connection (though somewhat loose) between anion binding strength and transport activity. The most powerful anionophores (e.g.7–9) possess multiple hydrogen bond donor groups and, in closely-related structures, it is usually found that affinities correlate with transport rates. Secondly lipophilicity plays a key role, if only to ensure that the carrier is located in the membrane.11,12,15,23 Support for these generalisations was recently obtained from a QSAR study, which also suggested an inverse correlation between size and effectiveness.11 Finally, there is evidence that structures which encapsulate the anionic substrate may be favoured.24
While these guidelines are helpful, there are still many variations in anionophore performance that are difficult to explain. One example emerged from studies of diureido decalins 8.21 These carriers were prepared in three variants, with side-chain R = Me, Et and octyl. Interestingly, activities were found to increase quite substantially in this order. On one level this correlation between activity and lipophilicity might have been anticipated, as the octyl derivative is more reliably confined to the membrane (see above). However, in this case all variants were highly lipophilic. Even the Me derivatives were expected to locate exclusively in the membrane interior, and indeed were shown to do so.25 Lipophilicity might confer some benefit beyond partitioning. However in cholapods 9, a major lengthening of side-chain R (from CH3 to C20H41) yielded no improvement.8 This implied that, once the transporter is in the membrane, the effect of added hydrocarbon might not be straightforward. In particular it suggested that overall lipophilicity might be less important than achieving a particular distribution of lipophilic groups within the molecule.
Resolving such issues in the powerful but complex anionophores 8 and 9 would be difficult, so we turned to the simple thioureas 2 (X = S). These are easily varied and, though not especially active, show readily measurable chloride–nitrate exchange in synthetic vesicles. They have previously been used to show the effects of hydrogen bond donor strength and overall lipophilicity on anionophore activity.11 Here we report a study which focuses on variants in which both these factors are kept constant. The results suggest a new design principle for anion carriers, namely that the anion-binding site should be placed centrally within the scaffold, surrounded by a balanced array of lipophilic substituents.
Results and discussion
Experiment design and procedures
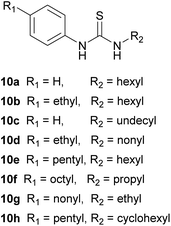
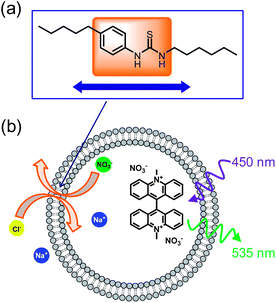
Our plan was to test a series of N-arylthioureas of general form 10, where R1 and R2 = H or alkyl. The hydrogen bond donor strength of these systems should be very similar, while the lipophilicities should vary according to the total number of carbon atoms in R1 + R2 (Ctotal). By keeping this number constant, we could generate a series with almost identical binding strength and lipophilicity, and would thus be able to probe solely the effect of binding site position (Fig. 2a). To simplify interpretation of the transport data, we preferred to choose R1 + R2 such that the transporters were confined to the membrane. In this case concentrations within the membrane could be known for certain, and the data could be guaranteed to reflect intrinsic transport ability. An initial study was thus required to determine Ctotal, as described below.To measure transport effectiveness, we focused mainly on the “lucigenin method” (Fig. 2b).1f,22 Briefly, solutions of the lipids 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and cholesterol in chloroform (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio) were mixed with a solution of N-arylthiourea transporter in methanol, so that the transporter to lipid ratio was 1
3 ratio) were mixed with a solution of N-arylthiourea transporter in methanol, so that the transporter to lipid ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250. The organic solvents were removed and the pre-mixed transporter/lipid film was rehydrated with an aqueous solution of 0.8 mM lucigenin and 225 mM sodium nitrate. The vesicles were then extruded through a membrane with 200 nm pores and external lucigenin was removed by size exclusion chromatography. The vesicles were diluted with 225 mM aqueous sodium nitrate solution to a final lipid concentration of 0.4 mM. Upon addition of 25 mM sodium chloride to the bulk solution the N-arylthiourea started to exchange chloride for nitrate across the vesicle membrane (see Fig. 2b). Quenching of lucigenin fluorescence by incoming chloride caused a decay in fluorescence output, which was measured over time. The normalized and averaged fluorescence curves were analysed to obtain two quantitative measures of transport effectiveness. Fitting to a double-exponential decay function produced accurate reproductions of the curves, from which initial rates I (changes in relative fluorescence, F/F0) were calculated. Fitting to a single exponential decay allowed the effective half-lives t½ to be estimated.8 Further details on the preparation of vesicles, transport measurements, and fitting of the data are given in the ESI.†
250. The organic solvents were removed and the pre-mixed transporter/lipid film was rehydrated with an aqueous solution of 0.8 mM lucigenin and 225 mM sodium nitrate. The vesicles were then extruded through a membrane with 200 nm pores and external lucigenin was removed by size exclusion chromatography. The vesicles were diluted with 225 mM aqueous sodium nitrate solution to a final lipid concentration of 0.4 mM. Upon addition of 25 mM sodium chloride to the bulk solution the N-arylthiourea started to exchange chloride for nitrate across the vesicle membrane (see Fig. 2b). Quenching of lucigenin fluorescence by incoming chloride caused a decay in fluorescence output, which was measured over time. The normalized and averaged fluorescence curves were analysed to obtain two quantitative measures of transport effectiveness. Fitting to a double-exponential decay function produced accurate reproductions of the curves, from which initial rates I (changes in relative fluorescence, F/F0) were calculated. Fitting to a single exponential decay allowed the effective half-lives t½ to be estimated.8 Further details on the preparation of vesicles, transport measurements, and fitting of the data are given in the ESI.†
To check for variations in the “constant” parameters, we also measured the binding strengths and lipophilicities of the thioureas. Binding constants (Ka) to Bu4N+Cl− in CDCl3 were determined by 1H NMR titration, while lipophilicities were assessed by reverse phase HPLC using a Kinetex C18 column and a gradient of 50% methanol in water to 100% methanol as mobile phase.
Choice of Ctotal: leaching of thioureas from bilayer membranes
As discussed above, the interpretation of this study would be more secure if the transporters were known to reside solely in the membranes, so that loadings could be accurately known. Accordingly, we performed a preliminary study on thioureas 10a, 10b, and 10e (R2 = C6H13; R1 = H, C2H5, C5H11; Ctotal 6, 8 and 11 respectively). As expected, measured lipophilicities and transport efficiencies both increased in this order (Table 1, Fig. 3a), confirming the results of an earlier study involving these compounds.11| Compound | R1 | R2 | Total # aliphatic C atoms | Retention timea (min) | KCl− in CDCl3 (M−1)b | Initial rate I (s−1)c | t ½ (s)d | EC50 (mol% w.r.t lipid)e |
|---|---|---|---|---|---|---|---|---|
a Retention times on a reversed-phase HLPC, employed as an experimental measure of lipophilicity.
b Binding constants measured by 1H NMR titration of thiourea with Bu4N+Cl− in CDCl3.
c Initial rate and
d half life of Cl− influx mediated by thiourea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 thiourea to lipid ratio) into vesicles containing lucigenin, as measured by quenching of the fluorescence of lucigenin (see ESI for details).
e EC50 (at 270 s) from Hill plots to examine the Cl−/NO3− exchange in vesicles by these transporters as monitored using an ion selective electrode. 250 thiourea to lipid ratio) into vesicles containing lucigenin, as measured by quenching of the fluorescence of lucigenin (see ESI for details).
e EC50 (at 270 s) from Hill plots to examine the Cl−/NO3− exchange in vesicles by these transporters as monitored using an ion selective electrode.
|
||||||||
| 10a | H | C6H13 | 6 | 6.6 | 93 | 0.0014 | 275 | 2.7 |
| 10b | C2H5 | C6H13 | 8 | 9.1 | 47 | 0.0066 | 107 | 0.3 |
| 10c | H | C11H23 | 11 | 11.9 | 107 | 0.0056 | 116 | 5.3 |
| 10d | C2H5 | C9H19 | 11 | 11.7 | 44 | 0.0097 | 70.1 | 0.4 |
| 10e | C5H11 | C6H13 | 11 | 11.7 | 42 | 0.0203 | 39.5 | 0.08 |
| 10f | C8H17 | C3H7 | 11 | 11.9 | 52 | 0.0086 | 97.5 | 1.5 |
| 10g | C9H19 | C2H5 | 11 | 12.0 | 53 | 0.0038 | 161 | >10 |
| 10h | C5H11 | C6H11 | 11 | 11.1 | 30 | 0.0151 | 54.4 | 0.1 |
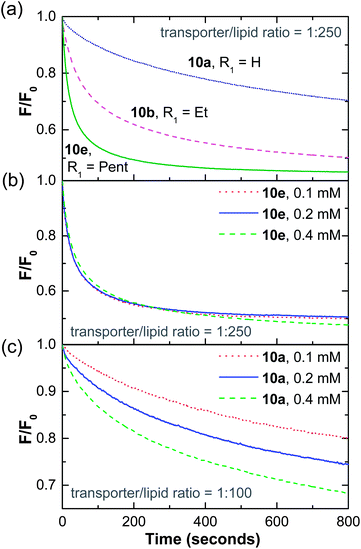
To check the distribution of these transporters between aqueous and lipid phases, we followed a previously reported procedure.21 Lucigenin-containing POPC/cholesterol vesicles were prepared with transporter present in the membrane as described before. However before testing for anion transport, the suspensions were diluted from 0.4 mM lipid to 0.2 mM. A further assay was then performed after dilution to 0.1 mM. If the transporter is confined to the membrane the traces at different concentrations should be superimposable after taking account of the dilution by normalisation. As shown in Fig. 3b, this was true to a good approximation for 10e. However, if the transporter partitions to any extent into the aqueous phase, dilution of the suspension should reduce the concentration in the bilayers. The overall effectiveness therefore becomes concentration-dependent. As shown in Figs. 3c and S30,† this is clearly the case for both 10a and 10b. For example, at [lipid] = 0.1 mM, 10a is less than half as active than at the original concentration of 0.4 mM. The differences between 10e, on the one hand, and 10a/b on the other, provided strong evidence for the validity of this test. They also established that (a) the activity trends in this series are due, at least in part, to differential partitioning between lipid and aqueous phases, and (b) that 10e with Ctotal = 11, was essentially confined to the membrane. This value was therefore adopted for the main part of the study.
11-Carbon atom series
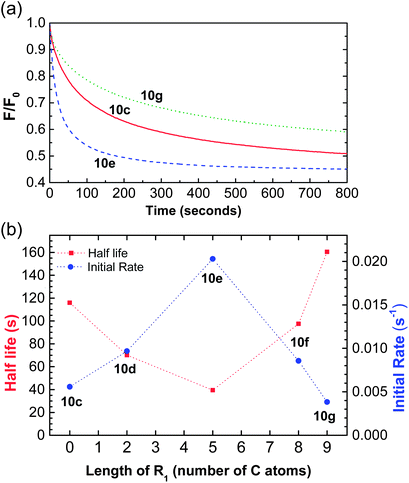
Having established that Ctotal should be 11, the thioureas 10c, 10d and 10f–h were prepared as further subjects for this study (see ESI†). Compounds 10c–g provide a sequence in which the thiourea moves from one end of the molecule to the other in steps of 2 or 3 carbons (cf.Fig. 2a), while 10h may be used to probe the effect of the shape of the lipophilic substituent. Representative molecules from this series (10c and 10g in addition to 10e) were tested for leaching as described above, with negative results (Fig. S30†). This allowed us to quantify the transport of chloride by these compounds, knowing that all transporter molecules were present in the lipid bilayer phase of the vesicles.Transport efficiencies for the new thioureas were assayed by the lucigenin method and compared to the results for 10e. Fluorescence decay traces for compounds 10c and 10g (at either end of the series) are shown in Fig. 4a, alongside that for 10e. It is immediately apparent that the position of the thiourea within the molecule has a very substantial effect; the transporter with a centrally-located anion-binding site is considerably more active than those with peripheral thioureas. Traces for the intermediate compounds 10d and 10f confirm the trend, showing intermediate effectiveness (Fig. S29†). The full set of transport data for 10c–g, quantified both as initial rate I and decay half-life t½, are summarised in Table 1 and presented graphically in Fig. 4b. By both measures, the central compound 10e is over twice as active as 10d and 10f, around 4 times as effective as 10c and more than 5 times as active as 10g.
To attribute this observed trend reliably to the positions of alkyl chains in compounds 10c–g, we had to exclude the effects caused by different binding constants or different overall lipophilicities. We therefore measured these parameters and the results are presented in Table 1. Binding constants (Ka) to Bu4N+Cl− in CDCl3 varied little for 10d–g lying between 42 and 53 M−1. Not unexpectedly, the affinity shown by 10c was slightly higher at 107 M−1; phenyl is more electron-withdrawing than p-alkylphenyl, leading to higher NH acidity. We should note however, that this higher affinity should improve its transport properties so presumably offsets the “position effect” discussed herein. Compound 10c is in fact slightly more active than 10g at the other end of the sequence, but is still one of the two least effective transporters. The lipophilicities of compounds 10c–g, as measured by HPLC, were very similar indeed; retention times all lay between 11.7 and 12 minutes.
As compound 10e gave rise to the fastest transmembrane anion transport, we were also interested to test its analogue 10h in which the linear hexyl chain at R2 was replaced by the more compact and rigid cyclohexyl. The change from linear to cyclic side-chain produced very little effect; compound 10h was marginally less active that 10e, but this could be accounted for by minor decreases in anion affinity and/or lipophilicity (see Table 1 for data).
Finally, anion transport by compounds 10a–h was also tested by a second procedure involving addition of carrier to preformed vesicles containing chloride, and measurement of Cl− efflux using an ion exchange electrode (ISE; see ESI for details†). The results were quantified as EC50 values, i.e. the concentration of transporter (in mole% with respect to lipid) required to elicit half the maximum observed response in the ISE signal at t = 270 seconds. As shown in Table 1, the values for compounds 10c–g followed the same pattern, with activity peaking at compound 10e.
Why does lipophilic balance matter?
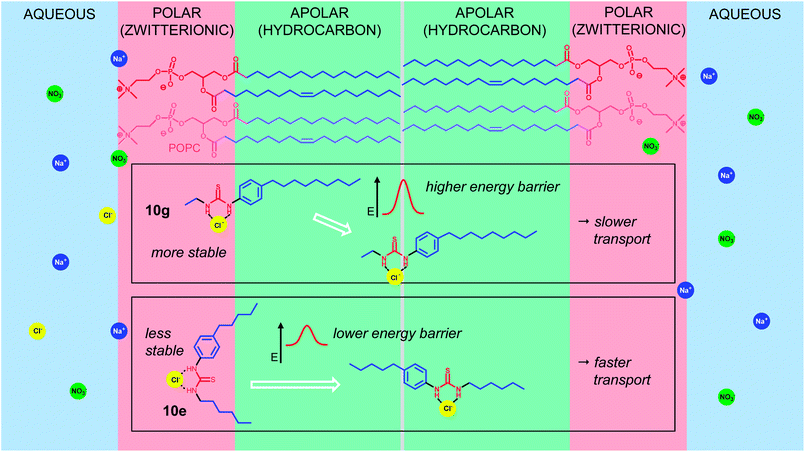
The results described above show clearly that the distribution of lipophilicity in anion transporters can have a substantial effect on activity. It seems that the preferred arrangement is to locate the anion-binding site at the centre of the molecule, i.e. with a balanced arrangement of lipophilic groups on either side. It is possible that this effect is due to differences in self-association within the membrane. However, this does not seem likely given that thioureas are not especially prone to aggregation, and that 10c–g are structurally very similar. Instead, we suggest that the role of lipophilic balance is to promote the transition between polar and apolar regions of the membrane bilayer. As shown schematically in Fig. 5, the POPC-based membrane may be divided into (i) the apolar hydrocarbon core and (ii) two polar surface regions dominated by the zwitterionic head-groups, flanked by aqueous phases containing the anionic substrates. Comparing compounds 10g and 10e, respectively the least and most active of the series, the former is quite close to a classic surfactant structure with polar head-group and lipophilic tail. If the N-ethylurea head-group binds a chloride ion from solution its polarity is greatly increased, creating a unit which sits comfortably in the hydrated polar region of the membrane.26 It is easily imagined that the transition from this environment to the apolar membrane interior could be quite unfavourable. In contrast the binding site of compound 10e, with substantial lipophilic groups on both sides, is less compatible with the polar surface regions. Having bound the target anion it has less incentive to stay in place and is better adapted to shepherd the anion through the membrane interior.Conclusions
It has been known for some time that the activity of anion carriers is determined not just by anion affinities and global lipophilicities, but also by other factors. However, until now, these other factors have remained ill-defined. The work described herein points clearly to a new factor or design principle, that of “lipophilic balance”. In a series of anionophores with closely similar affinities, sizes and lipophilicities, we have shown that variants with centralised binding sites, and thus a balanced arrangement of lipophilic groups, show greater effectiveness. The scale of the effect is considerable, and might be thought surprising. For example, moving four carbons from one side of the system to the other (as in 10g → 10e) can produce a >5-fold increase in activity. The results provide further confirmation that binding the target anion from water is only one aspect of anionophore activity, and that crossing the apolar barrier from one interface to the other is not straightforward. In future work we hope to uncover further principles that govern this process and can be used to design anionophores with potential for real-world applications.Acknowledgements
This work was supported by the Engineering and Physical Sciences Research Council (grant numbers EP/F03623X/1 and EP/J009687/1) and the European Cooperation in Science and Technology (COST) action CM1005 “Supramolecular Chemistry in Water”. PAG thanks the Royal Society and the Wolfson Foundation for a Royal Society Wolfson Research Merit Award.Notes and references
- (a) A. P. Davis, D. N. Sheppard and B. D. Smith, Chem. Soc. Rev., 2007, 36, 348–357 RSC; (b) G. W. Gokel and N. Barkey, New J. Chem., 2009, 33, 947–963 RSC; (c) J. T. Davis, O. Okunola and R. Quesada, Chem. Soc. Rev., 2010, 39, 3843–3862 RSC; (d) S. Matile, A. V. Jentzsch, J. Montenegro and A. Fin, Chem. Soc. Rev., 2011, 40, 2453–2474 RSC; (e) N. Busschaert and P. A. Gale, Angew. Chem., Int. Ed., 2013, 52, 1374–1382 CrossRef CAS PubMed; (f) H. Valkenier and A. P. Davis, Acc. Chem. Res., 2013, 46, 2898–2909 CrossRef CAS PubMed; (g) J. T. Davis, Top. Heterocycl. Chem., 2010, 24, 145–176 CAS; (h) P. A. Gale, R. Pérez-Tomás and R. Quesada, Acc. Chem. Res., 2013, 46, 2801–2813 CrossRef CAS PubMed.
- B. C. Pressman, Annu. Rev. Biochem., 1976, 45, 501–530 CrossRef CAS PubMed.
- F. M. Ashcroft, Ion Channels and Disease, Academic Press, London, 2000 Search PubMed.
- J. R. Broughman, L. P. Shank, W. Takeguchi, B. D. Schultz, T. Iwamoto, K. E. Mitchell and J. M. Tomich, Biochemistry, 2002, 41, 7350–7358 CrossRef CAS PubMed.
- (a) P. H. Schlesinger, R. Ferdani, J. Liu, J. Pajewska, R. Pajewski, M. Saito, H. Shabany and G. W. Gokel, J. Am. Chem. Soc., 2002, 124, 1848–1849 CrossRef CAS PubMed; (b) V. Sidorov, F. W. Kotch, G. Abdrakhmanova, R. Mizani, J. C. Fettinger and J. T. Davis, J. Am. Chem. Soc., 2002, 124, 2267–2278 CrossRef CAS PubMed; (c) N. Sakai, D. Houdebert and S. Matile, Chem.–Eur. J., 2003, 9, 223–232 CrossRef CAS PubMed; (d) R. E. Dawson, A. Hennig, D. P. Weimann, D. Emery, V. Ravikumar, J. Montenegro, T. Takeuchi, S. Gabutti, M. Mayor, J. Mareda, C. A. Schalley and S. Matile, Nat. Chem., 2010, 2, 533–538 CrossRef CAS PubMed; (e) V. Gorteau, G. Bollot, J. Mareda, A. Perez-Velasco and S. Matile, J. Am. Chem. Soc., 2006, 128, 14788–14789 CrossRef CAS PubMed; (f) C. R. Yamnitz, S. Negin, I. A. Carasel, R. K. Winter and G. W. Gokel, Chem. Commun., 2010, 46, 2838–2840 RSC; (g) X. Li, B. Shen, X. Q. Yao and D. Yang, J. Am. Chem. Soc., 2007, 129, 7264–7265 CrossRef CAS PubMed; (h) B. Shen, X. Li, F. Wang, X. Q. Yao and D. Yang, PLoS One, 2012, 7, e34694 CAS.
- (a) B. A. McNally, E. J. O'Neil, A. Nguyen and B. D. Smith, J. Am. Chem. Soc., 2008, 130, 17274–17275 CrossRef CAS PubMed; (b) A. Hennig, L. Fischer, G. Guichard and S. Matile, J. Am. Chem. Soc., 2009, 131, 16889–16895 CrossRef CAS PubMed.
- A. V. Koulov, T. N. Lambert, R. Shukla, M. Jain, J. M. Boon, B. D. Smith, H. Y. Li, D. N. Sheppard, J. B. Joos, J. P. Clare and A. P. Davis, Angew. Chem., Int. Ed., 2003, 42, 4931–4933 CrossRef CAS PubMed.
- B. A. McNally, A. V. Koulov, T. N. Lambert, B. D. Smith, J. B. Joos, A. L. Sisson, J. P. Clare, V. Sgarlata, L. W. Judd, G. Magro and A. P. Davis, Chem.–Eur. J., 2008, 14, 9599–9606 CrossRef CAS PubMed.
- (a) N. J. Andrews, C. J. E. Haynes, M. E. Light, S. J. Moore, C. C. Tong, J. T. Davis, W. A. Harrell and P. A. Gale, Chem. Sci., 2011, 2, 256–260 RSC; (b) N. Busschaert, P. A. Gale, C. J. E. Haynes, M. E. Light, S. J. Moore, C. C. Tong, J. T. Davis and W. A. Harrell Jr, Chem. Commun., 2010, 46, 6252–6254 RSC; (c) N. Busschaert, M. Wenzel, M. E. Light, P. Iglesias-Hernández, R. Pérez-Tomás and P. A. Gale, J. Am. Chem. Soc., 2011, 133, 14136–14148 CrossRef CAS PubMed; (d) N. Busschaert, L. E. Karagiannidis, M. Wenzel, C. J. E. Haynes, N. J. Wells, P. G. Young, D. Makuc, J. Plavec, K. A. Jolliffe and P. A. Gale, Chem. Sci., 2014 10.1039/C3SC52006D.
- S. J. Moore, M. Wenzel, M. E. Light, R. Morley, S. J. Bradberry, P. Gomez-Iglesias, V. Soto-Cerrato, R. Perez-Tomas and P. A. Gale, Chem. Sci., 2012, 3, 2501–2509 RSC.
- N. Busschaert, S. J. Bradberry, M. Wenzel, C. J. E. Haynes, J. R. Hiscock, I. L. Kirby, L. E. Karagiannidis, S. J. Moore, N. J. Wells, J. Herniman, G. J. Langley, P. N. Horton, M. E. Light, I. Marques, P. J. Costa, V. Felix, J. G. Frey and P. A. Gale, Chem. Sci., 2013, 4, 3036–3045 RSC.
- C. J. E. Haynes, N. Busschaert, I. L. Kirby, J. Herniman, M. E. Light, N. J. Wells, I. Marques, V. Felix and P. A. Gale, Org. Biomol. Chem., 2014, 12, 62–72 CAS.
- A. V. Jentzsch, D. Emery, J. Mareda, S. K. Nayak, P. Metrangolo, G. Resnati, N. Sakai and S. Matile, Nat. Commun., 2012, 3, 905 CrossRef PubMed.
- L. Adriaenssens, C. Estarellas, A. V. Jentzsch, M. M. Belmonte, S. Matile and P. Ballester, J. Am. Chem. Soc., 2013, 135, 8324–8330 CrossRef CAS PubMed.
- C. J. E. Haynes, S. J. Moore, J. R. Hiscock, I. Marques, P. J. Costa, V. Felix and P. A. Gale, Chem. Sci., 2012, 3, 1436–1444 RSC.
- N. Busschaert, I. L. Kirby, S. Young, S. J. Coles, P. N. Horton, M. E. Light and P. A. Gale, Angew. Chem., Int. Ed., 2012, 51, 4426–4430 CrossRef CAS PubMed.
- P. V. Santacroce, J. T. Davis, M. E. Light, P. A. Gale, J. C. Iglesias-Sanchez, P. Prados and R. Quesada, J. Am. Chem. Soc., 2007, 129, 1886–1887 CrossRef CAS PubMed.
- J. T. Davis, P. A. Gale, O. A. Okunola, P. Prados, J. C. Iglesias-Sanchez, T. Torroba and R. Quesada, Nat. Chem., 2009, 1, 138–144 CrossRef CAS PubMed.
- S. Bahmanjah, N. Zhang and J. T. Davis, Chem. Commun., 2012, 48, 4432–4434 RSC.
- S. J. Moore, C. J. E. Haynes, J. Gonzalez, J. L. Sutton, S. J. Brooks, M. E. Light, J. Herniman, G. J. Langley, V. Soto-Cerrato, R. Perez-Tomas, I. Marques, P. J. Costa, V. Felix and P. A. Gale, Chem. Sci., 2013, 4, 103–117 RSC.
- S. Hussain, P. R. Brotherhood, L. W. Judd and A. P. Davis, J. Am. Chem. Soc., 2011, 133, 1614–1617 CrossRef CAS PubMed.
- B. A. McNally, A. V. Koulov, B. D. Smith, J. B. Joos and A. P. Davis, Chem. Commun., 2005, 1087–1089 RSC.
- V. Saggiomo, S. Otto, I. Marques, V. Felix, T. Torroba and R. Quesada, Chem. Commun., 2012, 48, 5274–5276 RSC.
- L. W. Judd and A. P. Davis, Chem. Commun., 2010, 46, 2227–2229 RSC.
- In the case of bis-trifluoromethylphenylurea 8c (R = Me).
- A similar arrangement, supported by UV-vis spectra, has been proposed for N-alkyl-N′-p-nitrophenylthioureas in DDAB cationic vesicles. See: T. Hayashita, T. Onodera, R. Kato, S. Nishizawa and N. Teramae, Chem. Commun., 2000, 755–756 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterisation of the new transporter compounds 10c, d, f–h; experimental and analysis details for 1H NMR titrations; experimental details for measurement of chloride transport by the lucigenin assay, with additional fluorescence plots; experimental details of ion-selective electrode (ISE) measurements and Hill plots; details of lipophilicity assessments by reverse-phase HPLC. See DOI: 10.1039/c3sc52962b |
| This journal is © The Royal Society of Chemistry 2014 |