Benzenesulfonyl chlorides: new reagents for access to alternative regioisomers in palladium-catalysed direct arylations of thiophenes†
Kedong
Yuan
and
Henri
Doucet
*
Institut des Sciences Chimiques de Rennes, UMR 6226 CNRS-Université de Rennes 1, Organométalliques: Matériaux et Catalyse, Campus de Beaulieu, 35042 Rennes, France. E-mail: henri.doucet@univ-rennes1.fr; Fax: +33 0223236939
First published on 3rd October 2013
Abstract
The palladium-catalysed coupling of benzenesulfonyl chlorides with thiophene derivatives allows regioselective access to β-arylated thiophenes. The reaction proceeds with easily accessible catalyst, base and substrates, without oxidant or ligand and tolerates a variety of substituents on both the benzene and thiophene moieties.
Introduction
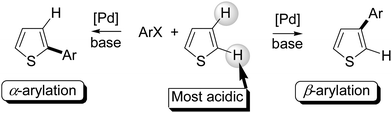
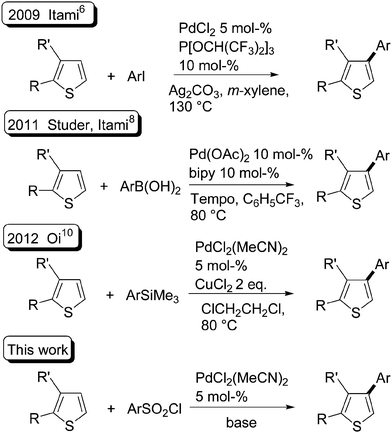
In recent years, metal-catalysed functionalization of C–H bonds has emerged as a powerful method for simpler access to molecules useful for materials or biological applications. If specific C–H bonds of (hetero)arenes can be easily coupled with arenes, it becomes one of the simplest methods to access bi(hetero)arenes.1 From thiophene derivatives and aryl halides as the coupling partners, the α-arylated products were easily obtained using palladium catalysts under various conditions (Fig. 1, left).1g,2 On the other hand, until recently, relatively little effort has been expended towards developing such metal-catalysed direct arylation reactions for the synthesis of β-arylated thiophenes (Fig. 1, right).3A few examples of such β-arylations3 have been reported using new catalysts, reactants and/or reaction conditions.4–9 Some of them employ a directing group on the thiophene derivative such as a 2-pyridyl or a carboxanilide function to control the regioselectivity of the arylation.4,5 In 2009, Itami and co-workers established a catalytic system that promotes the β-selective arylation of thiophene derivatives with aryl iodides without a directing group on the thiophenes. This β-functionalisation was obtained using PdCl2 associated with a phosphite ligand using Ag2CO3 as the base (Scheme 1, top).6 Glorius and co-workers have very recently reported an heterogeneously catalyzed C–H arylation of benzo[b]thiophenes.7 They demonstrated that, using Pd/C catalyst without additional ligands or directing groups on benzothiophene, but in the presence of 10 mol% CuCl, the less reactive β-position of benzothiophenes was arylated with excellent selectivity. Studer and Itami have also developed a method for the β-arylation of thiophenes with arylboronic acids under Pd/TEMPO catalysis (Scheme 1, middle).8 The same year, Bach et al. reported that thiophenes substituted at C3 by CH2COOEt or CH2PO(OEt)2 undergo a regioselective oxidative coupling reaction at C4 with various arylboronic acids in the presence of silver oxide, cesium trifluoroacetate [Cs(tfa)], benzoquinone (BQ), and Pd(tfa)2 catalyst in trifluoroacetic acid.9 In 2012, Oi et al. described the direct arylation of (benzo)thiophenes with aryltrimethylsilanes. The use of PdCl2(MeCN)2 catalyst in the presence of CuCl2 as oxidant also gives β-arylated thiophenes (Scheme 1, middle).10
 | ||
| Scheme 1 Procedures for Pd-catalysed β-arylations of thiophene derivatives without directing groups. | ||
In 2009, Dong and co-workers reported the Pd-catalysed coupling of 2-phenylpyridine with benzenesulfonyl chlorides to prepare sulfones.11 However, in the course of this study they also observed, in one case, a desulfitative12 direct arylation of a quinoline derivative when using elevated temperature in the presence of Ag2CO3 and CuBr. The use of benzenesulfonyl chlorides13–15 as the coupling partners for the palladium-catalysed desulfitative direct arylation of benzoxazoles derivatives has been recently described by Cheng et al.16 Using 10 mol% Pd(OAc)2 catalyst, K2CO3 as base and 1 equiv. of CuI as additive, the 2-arylbenzoxazoles were obtained in high yields. On the other hand, to the best of our knowledge, the desulfitative direct arylation of thiophenes with benzenesulfonyl chlorides has not been reported.
Results and discussion
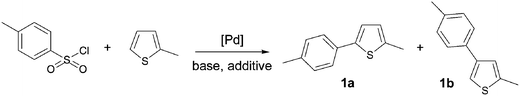
Herein, we describe a novel route to β-arylated thiophene derivatives using desulfitative palladium-catalysed C–H functionalisation, from thiophenes and benzenesulfonyl chlorides as the coupling partners (Scheme 1, bottom). The influence of both the benzenesulfonyl chloride and thiophene substituents is reported.We first examined the influence of several reaction conditions on the product formation (Table 1). The reaction of 4-methylbenzenesulfonyl chloride with 2-methylthiophene using Pd(OAc)2 catalyst and K2CO3 as the base only gave decomposed benzenesulfonyl chloride (Table 1, entry 1). The use of PdCl(C3H5)(dppb) or PdCl2 catalysts led to the desired product 1b in trace quantities (Table 1, entries 2 and 3). On the other hand, Pd(PhCN)2Cl2 catalyst in the presence of a mixture of K2CO3/LiCl gave product 1b in 30% yield (Table 1, entry 4). The addition of 1 equiv. of CuI to the reaction mixture or the use of DMSO or toluene as the solvents resulted in very low yields of 1b. The reaction temperature was found to be a crucial factor for such couplings, as the use of 140 °C instead of 120 °C allowed to increase the yield of 1b to 75% (Table 1, entry 8). Moreover, an excellent regioselectivity was observed, as 1b was produced to a level of 99%. The desired product 1b was obtained in 43% yield using Li2CO3 without additive in the presence of Pd(PhCN)2Cl2 catalyst; whereas similar conditions using Pd(MeCN)2Cl2 catalyst gave 1b in 80% yield (Table 1, entries 9 and 10). Then, we examined the influence of several bases at 140 °C using Pd(MeCN)2Cl2 catalyst. Lower yields of 1b were obtained using three equiv. of K3PO4, KOAc, Na2CO3 or K2CO3 (Table 1, entries 11–14). A complete decomposition of 4-methylbenzenesulfonyl chloride, without formation of 1b, was observed in the presence of Cs2CO3 (Table 1, entry 15). This difference between carbonated bases might be due to the higher solubility of Cs2CO3 compared with Li2CO3, Na2CO3 or K2CO3 in dioxane. A longer reaction time allowed to increase the yield of 1b to 84% with complete conversion of 4-methylbenzenesulfonyl chloride (Table 1, entry 17). Finally, the influence of a few other solvents was examined. NMP, DMF and DMSO gave only decomposed benzenesulfonyl chloride (Table 1, entries 18–20); whereas ethylbenzene and xylene gave 1b in very low yields (Table 1, entries 21 and 22).
| Entry | Catalyst | Base (equiv.) | Additive (equiv.) | Solvent | Temp. (°C) | Yield in 1b (%) |
|---|---|---|---|---|---|---|
| a Conditions: [Pd] 5 mol%, 4-methylbenzenesulfonyl chloride (1 equiv.), 2-methylthiophene (1.5 equiv.), yield determined by GC and crude 1H NMR, 20 h, yields in parenthesis are isolated 1b. b 40 h. | ||||||
| 1 | Pd(OAc)2 | K2CO3 (2) | — | Dioxane | 120 | 0 |
| 2 | PdCl(C3H5)(dppb) | K2CO3 (2) | — | Dioxane | 120 | <5 |
| 3 | PdCl2 | K2CO3 (2) | LiCl (4) | Dioxane | 120 | <5 |
| 4 | Pd(PhCN)2Cl2 | K2CO3 (2) | LiCl (4) | Dioxane | 120 | 30 (25) |
| 5 | Pd(MeCN)2Cl2 | K2CO3 (2) | LiCl (1)/CuI (1) | Dioxane | 120 | <5 |
| 6 | Pd(PhCN)2Cl2 | K2CO3 (2) | LiCl (1) | DMSO | 120 | 0 |
| 7 | Pd(PhCN)2Cl2 | K2CO3 (2) | LiCl (1) | Toluene | 120 | <5 |
| 8 | Pd(PhCN)2Cl2 | K2CO3 (3) | LiCl (2) | Dioxane | 140 | 75 (71) |
| 9 | Pd(PhCN)2Cl2 | Li2CO3 (2) | — | Dioxane | 140 | 43 (40) |
| 10 | Pd(MeCN)2Cl2 | Li2CO3 (3) | — | Dioxane | 140 | 80 (62) |
| 11 | Pd(MeCN)2Cl2 | K3PO4 (3) | — | Dioxane | 140 | 48 |
| 12 | Pd(MeCN)2Cl2 | KOAc (3) | — | Dioxane | 140 | 56 |
| 13 | Pd(MeCN)2Cl2 | Na2CO3 (3) | — | Dioxane | 140 | 41 |
| 14 | Pd(MeCN)2Cl2 | K2CO3 (3) | — | Dioxane | 140 | 31 |
| 15 | Pd(MeCN)2Cl2 | Cs2CO3 (3) | — | Dioxane | 140 | 0 |
| 16 | Pd(MeCN)2Cl2 | Li2CO3 (6) | — | Dioxane | 140 | 62 (60)b |
| 17 | Pd(MeCN)2Cl2 | Li2CO3 (3) | — | Dioxane | 140 | 84 (75)b |
| 18 | Pd(MeCN)2Cl2 | Li2CO3 (3) | NMP | 140 | 0 | |
| 19 | Pd(MeCN)2Cl2 | Li2CO3 (3) | DMF | 140 | 0 | |
| 20 | Pd(MeCN)2Cl2 | Li2CO3 (3) | DMSO | 140 | 0 | |
| 21 | Pd(MeCN)2Cl2 | Li2CO3 (3) | Ethylbenzene | 140 | <5% | |
| 22 | Pd(MeCN)2Cl2 | Li2CO3 (3) | — | p-Xylene | 140 | <5% |
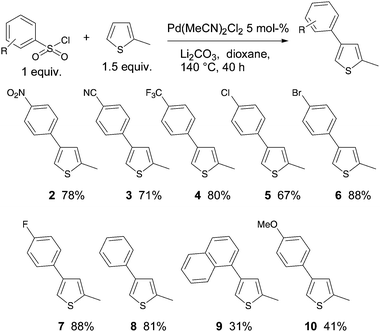
Then, the scope of the substituents on the benzenesulfonyl chloride moiety for reaction with 2-methylthiophene was examined using 5 mol% Pd(MeCN)2Cl2 catalyst in the presence of Li2CO3 (Scheme 2). We initially employed electron-deficient benzenesulfonyl chlorides. Nitro- and cyano-substituents at C4 of benzenesulfonyl chlorides gave regioselectively (>98%) the 4-arylated 2-methylthiophenes 2 and 3 in 78% and 71% yields, respectively. It should be noted that from 4-trifluoromethylbenzenesulfonyl chloride the β-arylation product 3 was only obtained with 92% regioselectivity.
Good yields and high regioselectivities (98%) were also obtained for the coupling of 4-chloro and 4-bromo-benzenesulfonyl chlorides with 2-methylthiophene, as the desired products 5 and 6 were isolated in 67% and 81% yields, respectively. It should be noted that for these two reactions, no cleavage of the C–Cl and C–Br bonds was observed allowing further transformations. A good yield of 88% of 7 was also obtained from the slightly electron-deficient 4-fluorobenzenesulfonyl chloride. On the other hand, poor yields of 9 and 10 were produced from the congested naphthalene-1-sulfonyl chloride and from the electron-rich 4-methoxybenzenesulfonyl chloride.
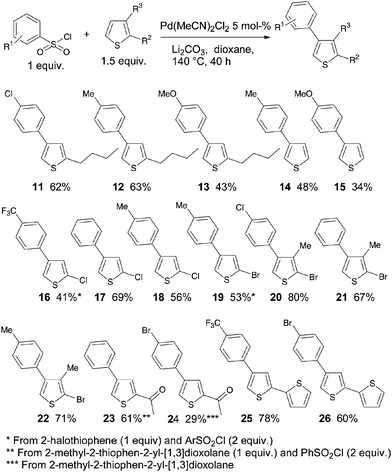
The influence of the substituents at C2 on the thiophene moiety was also evaluated (Scheme 3). Three benzenesulfonyl chlorides were coupled with 2-n-butylthiophene. Again, satisfactory yields of 11 and 12 were obtained in the presence of 4-chloro or 4-methyl substituents on the benzene ring, whereas a 4-methoxy substituent led to a poor yield of 13. The arylation of unsubstituted thiophene was also found to proceed at the β-position. However, moderate yields of 14 and 15 were obtained due to some formation of 3,4-diarylated thiophenes. Both chloro- and bromo-substituents at C2 of thiophene were also tolerated, and the 4-arylated 2-halothiophenes 16–22 were obtained in moderate to high yields. Even the use of protected 2-acetylthiophene led to the desired products 23 and 24 with high regioselectivity and moderate to good yields. The reaction of 4-trifluoromethyl- or 4-bromo-benzenesulfonyl chlorides with [2,2′]bithiophenyl also afforded the desired β-arylated compounds 25 and 26 in 78% and 60% yields, respectively.
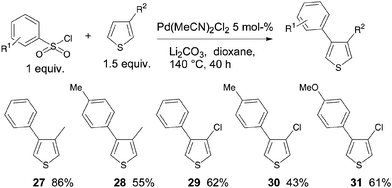
The 4-arylation of 3-substituted thiophenes was also found to proceed under these reaction conditions (Scheme 4). We first examined the reactivity of 3-methylthiophene in the presence of two benzenesulfonyl chlorides. A high yield of 86% of 27 was obtained using benzenesulfonyl chloride; whereas from 4-methylbenzenesulfonyl chloride, 28 was isolated in only 55% yield. The coupling of benzenesulfonyl chlorides with 3-chlorothiophene also produced the desired 4-arylated 3-chlorothiophenes 29–31. It should be noted that no cleavage of the C–Cl thiophene bond was observed.
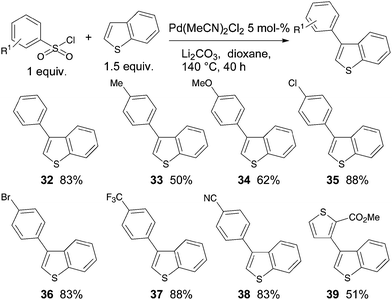
Finally, we examined the regioselectivity of the arylation of benzothiophene (Scheme 5). Glorius et al. have recently reported that benzothiophenes can be arylated at the β-position with aryl chlorides using an heterogeneous palladium catalyst associated to copper.7
Using our reaction conditions, in all cases the β-aryl-benzothiophenes were regioselectively produced. Again the best yields were obtained in the presence of electron-deficient benzenesulfonyl chlorides. For example, the use of 4-chloro- or 4-trifluoromethyl-benzenesulfonyl chlorides led to 35 and 37 in 88% yields, respectively. Again, from 4-trifluoromethylbenzenesulfonyl chloride the α-arylation product was also detected with 8% selectivity by GC/MS analysis. Benzenesulfonyl chloride also reacts nicely with benzothiophene to give 32 in 83% yield. It is worth noting that 4-bromobenzenesulfonyl chloride allows the formation of 36 in 83% yield. A reaction of benzothiophene with methyl 3-chlorosulfonylthiophene-2-carboxylate gave 39 in 51% yield, due to an α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β arylation ratio of 11
β arylation ratio of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 89; but without formation of bithiophene from self-coupling. This lower regioselectivity might arise from the steric hindrance of this chlorosulfonyl derivative.
89; but without formation of bithiophene from self-coupling. This lower regioselectivity might arise from the steric hindrance of this chlorosulfonyl derivative.
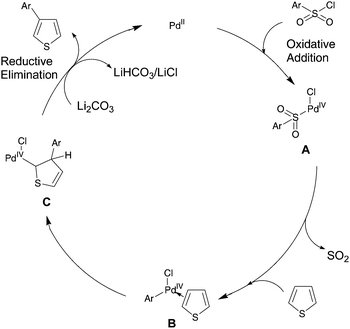
Although the mechanism cannot yet be elucidated, a catalytic cycle shown in Scheme 6 can be proposed. The first step of the catalytic cycle is probably the oxidative addition of the benzenesulfonyl chloride to Pd(II) to afford the Pd(IV) intermediate A, as in the reactions reported by Dong.11 Such oxidative additions to Pd(II) have been found to proceed even at room temperature.11b Then, after elimination of SO2, the coordination of thiophene gives B. The migration of the aryl group to the β-carbon atom of thiophene gives C. Finally, base-assisted proton abstraction gives the β-arylated thiophene and regenerates the Pd(II) species.
Experimental
General procedure for synthesis of arylated (benzo)thiophenes
To a 25 mL oven dried Schlenk tube, arylsulfonyl chloride (1 mmol), thiophene derivative (1.5 mmol), Li2CO3 (6 mmol, 0.444 g), 1,4-dioxane (2 mL) and bis(acetonitrile)dichloropalladium(II) (0.05 mmol, 12.9 mg) were successively added. The reaction mixture was evacuated by vacuum–argon cycles (5 times) and stirred at 140 °C (oil bath temperature) for 40 hours. After cooling the reaction to room temperature and concentration, the crude mixture was purified by silica column chromatography (Et2O/PE) to afford the C3 or C4 arylated products.Conclusions
In summary, we report here the first palladium-catalysed desulfitative arylation of thiophene derivatives. The reaction was found to provide, in most cases, very regioselectively (≥98%) the β-arylated thiophenes. The reaction proceeds with easily accessible ligand-free Pd(MeCN)2Cl2 catalyst and Li2CO3 as base and tolerates a wide variety of substituents both on the benzenesulfonyl chloride and thiophene moieties. Due to the wide availability of diversely functionalised benzenesulfonyl chlorides, such simple reaction conditions (no expensive base and ligand) should be very attractive to synthetic or materials chemists for access to β-arylthiophenes.Acknowledgements
We thank the Ministère de la Recherche for a fellowship to K. Y. and the Centre National de la Recherche Scientifique and “Rennes Metropole” for providing financial support.Notes and references
- (a) D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174–238 CrossRef CAS PubMed; (b) T. Satoh and M. Miura, Chem. Lett., 2007, 36, 200–205 CrossRef CAS; (c) L.-C. Campeau, D. R. Stuart and K. Fagnou, Aldrichimica Acta, 2007, 40, 35–41 CAS; (d) I. V. Seregin and V. Gevorgyan, Chem. Soc. Rev., 2007, 36, 1173–1193 RSC; (e) B.-J. Li, S.-D. Yang and Z.-J. Shi, Synlett, 2008, 949–957 CAS; (f) C. Lewis, R. G. Bergman and J. A. Ellman, Acc. Chem. Res., 2008, 41, 1013–1025 CrossRef PubMed; (g) F. Bellina and R. Rossi, Tetrahedron, 2009, 65, 10269–10310 CrossRef CAS PubMed; (h) L. Ackermann, R. Vicente and A. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792–9826 CrossRef CAS PubMed; (i) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094–5115 CrossRef CAS PubMed; (j) O. Daugulis, H.-Q. Do and D. Skabashov, Acc. Chem. Res., 2009, 42, 1074–1086 CrossRef CAS PubMed; (k) L. Joucla and L. Djakovitch, Adv. Synth. Catal., 2009, 351, 673–714 CrossRef CAS; (l) C.-L. Sun, B.-J. Li and Z.-J. Shi, Chem. Commun., 2010, 46, 677–685 RSC; (m) C. Fischmeister and H. Doucet, Green Chem., 2011, 13, 741–753 RSC; (n) L. Ackermann, Chem. Rev., 2011, 111, 1315–1345 CrossRef CAS PubMed; (o) L. McMurray, F. O'Hara and M. J. Gaunt, Chem. Soc. Rev., 2011, 40, 1885–1898 RSC; (p) N. Kuhl, M. N. Hopkinson, J. Wencel-Delord and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 10236–10254 CrossRef CAS PubMed; (q) J. Yamaguchi, A. D. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2012, 51, 8960–9009 CrossRef CAS PubMed; (r) S. R. Neufeldt and M. S. Sanford, Acc. Chem. Res., 2012, 45, 936–946 CrossRef CAS PubMed; (s) J. Wencel-Delord and F. Glorius, Nat. Chem., 2013, 5, 369–375 CrossRef CAS PubMed.
- For Pd-catalysed arylation at C5 of thiophenes with aryl halides: (a) A. Ohta, Y. Akita, T. Ohkuwa, M. Chiba, R. Fukunaga, A. Miyafuji, T. Nakata, N. Tani and Y. Aoyagi, Heterocycles, 1990, 31, 1951–1958 CrossRef CAS PubMed; (b) B. Liégault, I. Petrov, S. I. Gorelsky and K. Fagnou, J. Org. Chem., 2010, 75, 1047–1060 CrossRef PubMed.
- J. Roger, A. L. Gottumukkala and H. Doucet, ChemCatChem, 2010, 2, 20–40 CrossRef CAS.
- (a) T. Okazawa, T. Satoh, M. Miura and M. Nomura, J. Am. Chem. Soc., 2002, 124, 5286–5287 CrossRef CAS PubMed; (b) K. Si Larbi, H. Y. Fu, N. Laidaoui, K. Beydoun, M. Miloudi, D. El Abed, S. Djebbar and H. Doucet, ChemCatChem, 2012, 4, 815–823 CrossRef CAS.
- (a) S. Kirchberg, T. Vogler and A. Studer, Synlett, 2008, 2841–2845 CAS ; For similar reaction with Rh catalyst; (b) T. Vogler and A. Studer, Org. Lett., 2008, 10, 129–131 CrossRef CAS PubMed ; For similar reaction with PhMgBr and an Fe catalyst; (c) N. Yoshikai, S. Asako, T. Yamakawa, L. Ilies and E. Nakamura, Chem.–Asian J., 2011, 6, 3059–3065 CrossRef CAS PubMed ; For similar reaction with Ru catalyst; (d) R. K. Chinnagolla and M. Jeganmohan, Org. Lett., 2012, 14, 5246–5249 CrossRef CAS PubMed.
- (a) S. Yanagisawa, K. Ueda, H. Sekizawa and K. Itami, J. Am. Chem. Soc., 2009, 131, 14622–14623 CrossRef CAS PubMed; (b) K. Ueda, S. Yanagisawa, J. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2010, 49, 8946–8949 CrossRef CAS PubMed.
- D.-T. D. Tang, K. D. Collins and F. Glorius, J. Am. Chem. Soc., 2013, 135, 7450–7453 CrossRef CAS PubMed.
- (a) S. Kirchberg, S. Tani, K. Ueda, J. Yamaguchi, A. Studer and K. Itami, Angew. Chem., Int. Ed., 2011, 50, 2387–2391 CrossRef CAS PubMed; (b) K. Yamaguchi, J. Yamaguchi, A. Studer and K. Itami, Chem. Sci., 2012, 3, 2165–2169 RSC; (c) K. Yamaguchi, H. Kondo, J. Yamaguchi and K. Itami, Chem. Sci., 2013, 4, 3753–3757 RSC.
- (a) I. Schnapperelle, S. Breitenlechner and T. Bach, Org. Lett., 2011, 13, 3640–3643 CrossRef CAS PubMed; (b) I. Schnapperelle and T. Bach, ChemCatChem, 2013, 5, 3232–3236 CrossRef CAS.
- K. Funaki, T. Sato and S. Oi, Org. Lett., 2012, 14, 6186–6189 CrossRef CAS PubMed.
- (a) X. Zhao, E. Dimitrijevic and V. M. Dong, J. Am. Chem. Soc., 2009, 131, 3466–3467 CrossRef CAS PubMed; (b) X. Zhao and V. M. Dong, Angew. Chem., Int. Ed., 2011, 50, 932–934 CrossRef CAS PubMed; (c) For sulfonylations of quinolines using CuI as catalyst: Z. Wu, H. Song, X. Cui, C. Pi, W. Du and Y. Wu, Org. Lett., 2013, 15, 1270–1273 CrossRef CAS PubMed ; For reviews on Pd(II)/Pd(IV) catalysis; (d) K. Muniz, Angew. Chem., Int. Ed., 2009, 48, 9412–9423 CrossRef CAS PubMed; (e) A. J. Canty, Dalton Trans., 2009, 10409–10417 RSC; (f) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147–1169 CrossRef CAS PubMed.
- For desulfitative couplings using Ru catalysts: (a) N. Kamigata, M. Yoshikawa and T. Shimizu, J. Fluorine Chem., 1998, 87, 91–95 CrossRef CAS; (b) D. A. Nagib and D. W. C. MacMillan, Nature, 2011, 480, 224–228 CrossRef CAS PubMed.
- For a review on transition-metal mediated C–S bond activation: L. Wang, W. He and Z. Yu, Chem. Soc. Rev., 2013, 42, 599–621 RSC.
- For Heck type reaction with benzenesulfonyl chlorides: (a) M. Miura, H. Hashimoto, K. Itoh and M. Nomura, Tetrahedron Lett., 1989, 30, 975–976 CrossRef CAS; (b) M. Miura, H. Hashimoto, K. Itoh and M. Nomura, J. Chem. Soc., Perkin Trans. 1, 1990, 2207 RSC.
- For Stille, Negishi or Suzuki couplings with benzenesulfonyl chlorides: (a) S. R. Dubbaka and P. Vogel, J. Am. Chem. Soc., 2003, 125, 15292–15293 CrossRef CAS PubMed; (b) S. R. Dubbaka and P. Vogel, Org. Lett., 2004, 9, 95–98 CrossRef PubMed; (c) S. R. Dubbaka and P. Vogel, Angew. Chem., Int. Ed., 2005, 44, 7674–7684 CrossRef CAS PubMed.
- (a) M. Zhang, S. Zhang, M. Lui and J. Cheng, Chem. Commun., 2011, 47, 11522–11524 RSC ; For reactions with sodium sulfinates; (b) B. Liu, Q. Guo, Y. Cheng, J. Lan and J. You, Chem.–Eur. J., 2011, 17, 13415–13419 CrossRef CAS PubMed; (c) R. Chen, S. Liu, X. Liu, L. Yang and G.-J. Deng, Org. Biomol. Chem., 2011, 9, 7675–7679 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Procedures and 1H and 13C NMR spectra. See DOI: 10.1039/c3sc52420e |
| This journal is © The Royal Society of Chemistry 2014 |