Magnetic nanoparticles-supported tungstic acid (MNP-TA): an efficient magnetic recyclable catalyst for the one-pot synthesis of spirooxindoles in water
Abstract
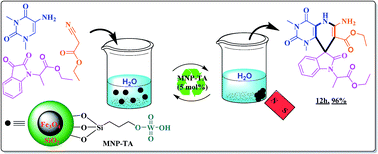
This paper reports the preparation, characterization and catalytic application of a novel, magnetically separable catalyst consisting of tungstic acid supported on silica coated magnetic nanoparticles. To obtain this new catalyst system, first, (3-chloropropyl)triethoxysilane was reacted with silica coated magnetic nanoparticles to generate a 3-chloropropyl magnetic nanoparticle (3-CPMNP) substrate. Subsequently, the addition of sodium tungstate to 3-CPMNP resulted in the stabilization of tungstic acid species on the surface of the magnetic nanoparticles (MNP-TA). The synthesized catalyst was characterized using some different microscopic and spectroscopic techniques such as X-ray powder diffraction (XRD), transmission electron microscopy (TEM), scanning electron microscopy (SEM), and FT-IR spectroscopy. The catalyst nanoparticles were obtained with near spherical morphology and an average size of ∼45 nm. The W content of the catalyst was determined by ICP analysis to be 82.5 ppm (82.5 mg L−1), which was equal to 8.25% w/w (0.45 mmol g−1). The catalyst was successfully used for the one-pot synthesis of spirooxindoles via the multicomponent reaction of isatins and dicarbonyl compounds in water, which was used as a green solvent. The results revealed that this new catalyst showed high catalytic activity in this protocol and that it can be reused at least 5 times without any change in its catalytic activity.

 Please wait while we load your content...
Please wait while we load your content...