Solar driven uphill conversion of dicyclopentadiene to cyclopentadiene: an important synthon for energy systems and fine chemicals†
Abstract
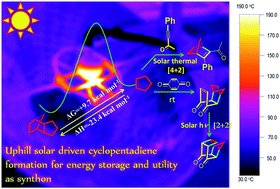
The retro Diels–Alder conversion of endo-dicyclopentadiene to cyclopentadiene (Cp) – which is thermodynamically uphill under ambient conditions (ΔG = +9.7 kcal mol−1; values based on computation at 273 K following CBS-QB3 methodology) – was carried out at 175–190 °C in neat state using solar energy. The reaction is thermodynamically favorable at elevated temperature. Considering heat release from the reverse reaction (ΔH = −23.4 kcal mol−1), the energy storage efficiency was computed to be ca. 5.5% with respect to the IR component in concentrated solar radiation. Solar energy was further utilized for preparation of a model 2,5-norbornadiene derivative (75% isolated yield) through the cycloaddition reaction of Cp with 4-phenylbut-3-yn-2-one at 150–185 °C. The norbornadiene–quadricyclane system has been proposed for solar energy storage, and its solar assisted synthesis would help reduce its carbon footprint over its life cycle. Norbornadiene synthesis using solar energy may also be of interest for greener processing of fuels derived from this compound. Cookson's cage ketone, which too has been proposed as an energy storage medium, was additionally synthesized from the Diels–Alder adduct of Cp and p-benzoquinone employing concentrated solar photo-thermochemical conditions. The reaction proceeded rapidly (15 min) and gave the desired product in 96% isolated yield. Besides the above applications, Cp is an important synthon in the preparation of fine chemicals.

 Please wait while we load your content...
Please wait while we load your content...