Structural incorporation of iron into Ge–imogolite nanotubes: a promising step for innovative nanomaterials†
Abstract
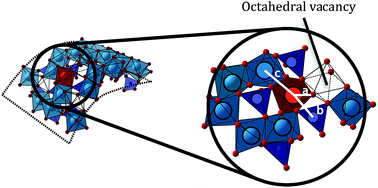
Novel iron-doped aluminogermanate nanotubes were obtained using a single step, aqueous phase synthesis protocol. These nanotubes are isostructural with imogolite, a natural occurring nanofiber, but are obtained by-product free in substantially larger quantities with aluminum substitution levels around 1%. Increasing the Fe concentrations led to higher substitution levels but also to the co-precipitation of Fe (oxy)hydroxides.

 Please wait while we load your content...
Please wait while we load your content...