Investigation of angiogenesis and its mechanism using zinc oxide nanoparticle-loaded electrospun tissue engineering scaffolds
Robin Augustine*a,
Edwin Anto Dominicb,
Indu Rejub,
Balarama Kaimalb,
Nandakumar Kalarikkal*ac and
Sabu Thomas*ad
aInternational and Inter University Centre for Nanoscience and Nanotechnology, Mahatma Gandhi University, Kottayam-686 560, Kerala, India. E-mail: robinaugustine9@gmail.com; Tel: +91-9562204140
bPushpagiri Research Centre, Pushpagiri Institute of Medical Sciences & Research Centre, Tiruvalla-689 101, Kerala, India
cSchool of Pure and Applied Physics, Mahatma Gandhi University, Kottayam-686 560, Kerala, India. E-mail: nkkalarikkal@mgu.ac.in; Fax: +91-481-2731669; Tel: +91-481-2731669
dSchool of Chemical Sciences, Mahatma Gandhi University, Kottayam-686 560, Kerala, India. E-mail: sabuchathukulam@yahoo.co.uk; Fax: +91-481-2731002; Tel: +91-481-2731002
First published on 26th September 2014
Abstract
Angiogenesis through tissue engineering scaffolds is an important factor that determines the success of a tissue engineering endeavor. Zinc oxide (ZnO) nanoparticles are well known for their ability to generate reactive oxygen species (ROS) which have a potential role in biological systems. ROS can induce angiogenesis through growth factor mediated mechanisms. Here, we report the fabrication of electrospun polycaprolactone scaffolds incorporated with ZnO nanoparticles and their ability to induce angiogenesis. This study demonstrated that the induction of angiogenesis was by the expression of key proangiogenic factors, fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF), upregulated due to the presence of ZnO nanoparticles. This is the first report suggesting the use of a metal/metal oxide nanoparticle in tissue engineering scaffolds to enhance angiogenesis.
1 Introduction
The formation of an active blood vessel network through an implanted scaffold is one of the most important issues in tissue engineering and regenerative medicine.1 An active blood vessel network is necessary for the integration of the scaffold with the existing host tissue.2 Formation of blood vessel takes place in scaffolds mainly by angiogenesis. Angiogenesis is the formation of new blood capillaries from existing blood vessels for supplying nutrients to cells that are distant from existing blood vessels.3 Angiogenesis is a complex process that is mediated by the endothelial cells that line blood vessels.4 Angiogenesis is a regulated process involving a number of stimulators such as fibroblast growth factor (FGF),5 vascular endothelial growth factor (VEGF),6 angiopoietins,7 activators of integrins,8 and inhibitors such as thrombospondin,9 angiostatin10 and endostatin.11 Even though the spontaneous vascularization of the implanted scaffold will take place, this is too slow to provide enough nutrient transport to the cells in the interior of the tissue engineered construct.2 Thus, to ensure the successful integration of the scaffold with the host tissue, additional strategies should be adopted. Although growth factors12 like VEGF are very effective regulators in tissue engineering to induce angiogenesis in scaffolds,13 their application for tissue regeneration must overcome numerous challenges, because their half-lives are only on the order of few minutes.14 Thus, the use of a material that can induce the cells to produce growth factors like FGF and VEGF may overcome these challenges.Role of reactive oxygen species (ROS) like hydrogen peroxide (H2O2) in the cell proliferation and wound healing through the activation of growth factors has been already reported.15–18 It is well established that ROS play an important role in angiogenesis by stimulating the key steps of cell proliferation, migration, and tube formation.19–23 In endothelial cells, especially, H2O2 is the most biologically important ROS that is involved in redox signaling events during angiogenesis. H2O2 enhanced the wound healing in mice by the expression of VEGF.16 H2O2 also induced FGF expression in rat astrocyte cells24 and retinal pigment epithelial (RPE) cells.25
Zinc oxide (ZnO) is an inorganic compound which is a generally recognized as safe (GRAS) material by the Food and Drug Administration and widely used in everyday applications. They can chemisorb oxygen as O2− taking electrons from the oxides26,27 and form H2O2 in presence of water.28 We therefore, hypothesized that the ZnO nanoparticles in the tissue engineering scaffold can play an important role in the generation of ROS, especially H2O2, which may trigger endothelial cell proliferation as well as the angiogenesis through the polymeric scaffolds.
Further, Ayan et al. reported that ZnO nanoflowers can induce proliferation and migration of endothelial cells and enhance angiogenesis.29 ROS driven angiogenesis by europium hydroxide [EuIII(OH)3] nanorods was also reported.30–33
Scaffolds are the most important components for tissue engineering.34 Polycaprolactone (PCL) has been used as a biomaterial for various biomedical applications including tissue engineering scaffolds, wound dressings, hemostats etc.35,36 Electrospinning of PCL, its blends and composites has been tried by many workers for tissue engineering applications.37–40
Our preliminary study had shown that electrospun PCL membranes containing ZnO nanoparticles improved the mechanical property of the membrane, imparted antibacterial property and had shown good biocompatibility in vitro.26 In vivo application as a skin substitute in guinea pigs demonstrated that these membranes were able to enhance healing of full thickness wounds without any scar formation due to the enhanced cell migration and proliferation.28
Thus, here we report the enhancement of angiogenic property of electrospun PCL tissue engineering scaffold by incorporating ZnO nanoparticles in the polymer matrix. Also, we demonstrated that the improved angiogenic and cell adhesion property were due to the expression of growth factors like FGF and VEGF by the H2O2 that may generated by ZnO nanoparticles. This will facilitates the successful integration host tissue with the scaffold and proliferating capillaries bring oxygen and micronutrients to growing tissues and remove catabolic waste products.
2 Experimental
2.1. Materials used
Polycaprolactone (Mw 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–90
000–90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and ZnO nanoparticles (≈60 nm) used in this study were purchased from Sigma Aldrich, USA. Acetone, hematoxylin monohydrate, eosin, NaCl and xylene were purchased from Merck, India. Lithium carbonate and mercuric oxide were obtained from Otto Chemie, India. Formaldehyde was purchased from Fisher Scientific, India. Adult human dermal fibroblast (HDFa) cells Dulbecco's modified eagles medium, fetal bovine serum, penicillin and streptomycin were purchased from Gibco, India. All the reagents used in this study were of analytical grade quality and therefore used without further purification.
000) and ZnO nanoparticles (≈60 nm) used in this study were purchased from Sigma Aldrich, USA. Acetone, hematoxylin monohydrate, eosin, NaCl and xylene were purchased from Merck, India. Lithium carbonate and mercuric oxide were obtained from Otto Chemie, India. Formaldehyde was purchased from Fisher Scientific, India. Adult human dermal fibroblast (HDFa) cells Dulbecco's modified eagles medium, fetal bovine serum, penicillin and streptomycin were purchased from Gibco, India. All the reagents used in this study were of analytical grade quality and therefore used without further purification.
2.2. Electrospinning of polycaprolactone with ZnO nanoparticles
Electrospinning parameters were selected based on our previous studies.26,28 The electrospinning setup was assembled by Holmarc, India and consisted of a syringe pump, a high voltage power supply and a 10 ml syringe with a 21G diameter needle. The tip to collector distance was maintained at 15 cm and a DC voltage of 18 kV was applied. The feed rate of the solution was precisely controlled by a syringe pumping system which was adjusted to a flow rate of 1 ml h−1. A thin aluminum sheet of 7 cm2 was used as the collector. Temperature and relative humidity inside the electrospinning chamber were 28 °C and 55% respectively.PCL membranes in which ZnO nanoparticles of particle size range ∼60 nm incorporated were prepared by electrospinning 15 wt% PCL in acetone. PCL solutions with varying concentration of ZnO nanoparticles were prepared in acetone. ZnO nanoparticles were accurately weighed and ultrasonicated for 15 minutes to homogeneously disperse in acetone. Then, known quantity of PCL was added into the above solution so that the final solution contains 15 wt% PCL and stirred in a magnetic stirrer for 12 h for the dissolution of the pellets and proper mixing. 10 ml of the prepared solutions with different wt% of ZnO nanoparticles were taken in plastic syringes and electrospun individually on aluminum sheets. Electrospinning was carried out for 10 hours to get scaffolds with a thickness of 1.2 ± 0.14 mm. After drying the membranes were peeled out from the aluminum sheets for further studies.
2.3. Characterization of scaffolds
The fabricated PCL/ZnO nanocomposite membranes were characterized for the morphology using SEM and FESEM. EDAX analysis was carried out to confirm the incorporation of ZnO nanoparticles in the scaffold. The angiogenic property was assessed using chicken chorioallantoic membrane (CAM) assay. Neovascularization was determined by the subcutaneous implantation of the membrane with different concentrations of ZnO nanoparticles.2.4. In vitro adult human dermal fibroblast cell seeding
Adult human dermal fibroblasts (HDFa), were cultured for 1 week in scaffolds with different amounts of ZnO nanoparticles, and neat PCL scaffolds with fibroblast culture medium. The electrospun mats were cut into 1 × 1 cm square shapes and both sides of the scaffold were sterilized by ultraviolet irradiation in a laminar flow hood for 30 min. Prior to cell seeding, the scaffolds were soaked in warm culture medium, dabbed gently on sterile blotting paper to remove excess moisture and placed in a 24-well plate. Cell pellet was suspended in culture medium and 30 μl of cell suspension containing 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells were pipetted on top of each scaffold. The plate was incubated at 37 °C for 40 min for cells to attach before 1000 μl of culture medium was added into each well. After the addition of medium, plates were incubated in an incubator with 5% CO2 supply at 38.5 °C for 1 week. After each 12 hours the culture plates were observed under an inverted phase contrast microscope (no. X51, Olympus, Japan) and images were taken using a CCD camera attached to the microscope. The whole experiments were done in triplicates. To get quantitative information regarding the number of cells present on the scaffolds, obtained images were analyzed using ImageJ software; number of cells per square millimeter area were calculated and expressed as the mean of 6 observations ± S.D. Statistical analysis has been carried out using Student's t-test. A P value less than 0.05 was considered as statistically significant.
000 cells were pipetted on top of each scaffold. The plate was incubated at 37 °C for 40 min for cells to attach before 1000 μl of culture medium was added into each well. After the addition of medium, plates were incubated in an incubator with 5% CO2 supply at 38.5 °C for 1 week. After each 12 hours the culture plates were observed under an inverted phase contrast microscope (no. X51, Olympus, Japan) and images were taken using a CCD camera attached to the microscope. The whole experiments were done in triplicates. To get quantitative information regarding the number of cells present on the scaffolds, obtained images were analyzed using ImageJ software; number of cells per square millimeter area were calculated and expressed as the mean of 6 observations ± S.D. Statistical analysis has been carried out using Student's t-test. A P value less than 0.05 was considered as statistically significant.
Fibroblast culture medium contained 89% Dulbecco's modified eagles medium (Gibco), 10% fetal bovine serum (Gibco) and 1% penicillin/streptomycin (Gibco); the medium was changed by 100% every 2–3 days during the 1 week culture.
2.5. Angiogenesis assay in chicken chorioallantoic membrane (CAM)
In order to determine the ability of polycaprolactone scaffolds containing ZnO nanoparticles to promote angiogenesis, CAM assay has been carried out. Briefly, fertilized chicken eggs were labeled and transferred into an egg incubator with a relative air humidity of 65% and a temperature of 37 °C. On embryo development day (EDD) 3, a hole of approximately 3 mm in diameter was made in the egg-shell and covered with a Parafilm® to prevent dehydration. The eggs were returned to the incubator in a static position until use. On EDD 8, the shell above the air pouch of the egg was extended to a diameter of approximately 3 cm in order to provide access to the chorioallantoic membrane. Then, 2 cm2 sized neat PCL membranes and with different concentrations of ZnO nanoparticles were deposited on the CAM. The eggs were labeled and returned to the incubator in a static position for 2 more days; photographs were taken and observed for angiogenesis. The whole experiments were carried out in triplicates. The blood vessel branch point at each area of the scaffold placement was counted manually in a clockwise direction. The results were expressed as mean with ±standard deviation. Statistical analysis has been carried out using Student's t-test. A P value less than 0.05 was considered as statistically significant.2.6. In vivo implantation study
To confirm the ability of scaffolds containing ZnO nanoparticles to induce angiogenesis in vivo, scaffolds were implanted in guinea pigs and histological examination of the scaffolds has been carried out at predetermined intervals. A brief experimental procedure is given as follows. 3 month-old American satin guinea pigs were housed in sterilized cages with sterile food and water and filtered air, and were handled with aseptic techniques. All the animal experiments were carried out in accordance with the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India with the approval of Institutional Animal Ethics committee, Pushpagiri Research Centre (PRC), Tiruvalla, India. Before implantation, all the scaffolds were treated with 70% alcohol for 20 minutes and then with ultraviolet radiation for 20 minutes to sterilize them. A small area on the left abdomen of the guinea pigs were shaved, wiped with alcohol, and anesthetized with Ketamine hydrochloride injection. A full thickness skin incision wound was made and a skin flap was reflected to expose the subcutaneous tissue. Neat PCL scaffolds and PCL scaffolds containing ZnO nanoparticles were implanted subcutaneously, and the flap was sutured to retain the membrane in the subcutaneous pouch. The skin pouch was surgically opened on day 5, 10 and 20 to evaluate the angiogenesis through the membranes. Membranes with attached subcutaneous tissue were excised for histological evaluation. The wound was sutured and then allowed to heal naturally. The whole procedure has been carried out in triplicates.The membranes collected from the animals were fixed in 10% formol saline and dehydrated in a graded series of ethanol, then embedded in paraffin wax. 3 micrometer-thick sections were cut with a rotary microtome (Leica 5 micron microtome). Hematoxylin and Eosin stained sections were investigated for the aggregation of RBCs and the formation of matured blood vessels. The stained sections were examined by at least two independent investigators with a Leica inverted light microscope.
2.7. Western analysis
HDFa cells were cultured on the scaffold as given in section 2.4. After 48 hours of cell culture, whole cell lysate was prepared and analyzed by western blotting. Scaffolds were washed twice in PBS, resuspended in 50 μl of sample buffer (10% glycerol, 5% mercaptoethanol, 0.0625 M Tris–HCl (pH 6.8), 2.5% SDS), and boiled for 15 mm. Centrifuged the lysed cells for 10 minutes at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–20
000–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g (4 °C) to pellet the cellular debris. 10 μg of total protein from each sample were run on 12% SDS-polyacrylamide gels and blotted onto nitrocellulose membranes. The membranes were pretreated with Tris-buffered saline (TBS) containing 0.1% Tween-20 and 5% dry milk and incubated at room temperature overnight in TBS buffer (TBS plus 0.1% Tween-20, 0.5% dry milk, and 0.1% fetal bovine serum) containing 0.05% (v/v) mouse Monoclonal Anti-VEGF-A antibody (Sigma Aldrich) and 1
000g (4 °C) to pellet the cellular debris. 10 μg of total protein from each sample were run on 12% SDS-polyacrylamide gels and blotted onto nitrocellulose membranes. The membranes were pretreated with Tris-buffered saline (TBS) containing 0.1% Tween-20 and 5% dry milk and incubated at room temperature overnight in TBS buffer (TBS plus 0.1% Tween-20, 0.5% dry milk, and 0.1% fetal bovine serum) containing 0.05% (v/v) mouse Monoclonal Anti-VEGF-A antibody (Sigma Aldrich) and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 dilution of mouse polyclonal Anti-FGF-2 antibody (Millipore, USA). After washing, the blots were incubated for 2 h with 0.033% (v/v) Anti-Mouse IgG (whole molecule)-peroxidase antibody conjugated to horseradish peroxidase (Sigma Aldrich), and the bound antibody was detected with the ECL system (Amersham). The membranes were reprobed with mouse monoclonal Anti-β-Actin antibody (Sigma Aldrich) to control for equal gel loading. The immune reaction was detected by exposing processed membranes to Kodak BIOMAX X-ray film.
1000 dilution of mouse polyclonal Anti-FGF-2 antibody (Millipore, USA). After washing, the blots were incubated for 2 h with 0.033% (v/v) Anti-Mouse IgG (whole molecule)-peroxidase antibody conjugated to horseradish peroxidase (Sigma Aldrich), and the bound antibody was detected with the ECL system (Amersham). The membranes were reprobed with mouse monoclonal Anti-β-Actin antibody (Sigma Aldrich) to control for equal gel loading. The immune reaction was detected by exposing processed membranes to Kodak BIOMAX X-ray film.
3 Results
3.1. Morphology of electrospun membranes
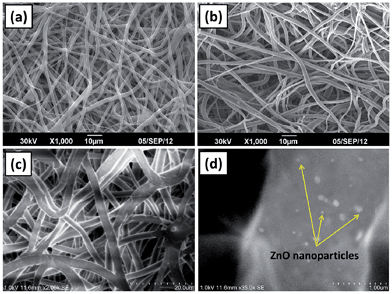
Representative morphological features of neat PCL scaffolds and its nanocomposite with ZnO nanoparticles are given in Fig. 1. It is evident from the figure that the fabricated scaffolds were highly porous with randomly oriented fibers. Lower magnification FESEM image of PCL scaffolds containing 1 wt% ZnO nanoparticles (Fig. 1(c)) shows that the surface of the individual fibers were smooth whereas the higher magnification micrograph confirms the presence of well dispersed nanoparticle on the fibers (Fig. 1(d)).3.2. Energy-dispersive X-ray spectroscopy (EDX)
The EDX spectrum of PCL and PCL/ZnO nano-composite scaffolds confirmed the presence of the ZnO nanoparticles entrapped within the PCL matrix. It also confirmed the ZnO nanoparticles reached the collector along with the polymer solution during the electrospinning process. Spectra of neat PCL scaffolds and ZnO nanoparticles incorporated PCL scaffolds at varying filler content are shown in Fig. 2. In the case of neat PCL there are some sharp low-energy peaks which are corresponding to the elements carbon and oxygen (Fig. 2(a)). While, in the case of PCL/ZnO composite scaffolds, three additional peaks could be observed at the energy levels 1 keV, 8.5 keV and 9 keV which are the characteristic of the zinc element. There is a remarkable increase in the intensity of the three characteristic peaks of zinc as well as one of oxygen while increasing the ZnO nanoparticle content from 1 to 4 wt% (Fig. 2(b) and (c)).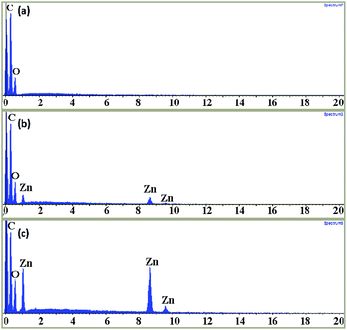 | ||
| Fig. 2 EDX (energy-dispersive X-ray spectrum) of neat PCL scaffold (a), PCL scaffold with 1 wt% (b) and 4 wt% (c) ZnO nanoparticle content. | ||
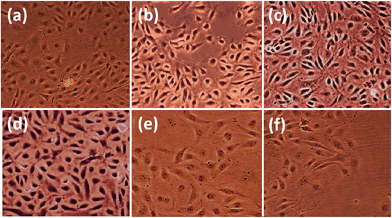
3.3. In vitro HDFa cell proliferation
We investigated the effect of incorporated ZnO nanoparticles on the growth of adult human dermal fibroblast (HDFa) cells in the PCL scaffolds. We compared cell growth over 1 week period in the scaffolds containing various concentrations of ZnO nanoparticles (0, 0.5, 1, 2, 4 and 6 wt%). We consistently noticed statistically (P ≤ 0.05) higher cell density at every period on the scaffolds with ZnO nanoparticles compared with the control scaffolds without nanoparticles (Fig. 3). This indicated that incorporated ZnO nanoparticles promoted greater HDFa cell proliferation on the PCL scaffold.As shown in Table 1, the cell density in the scaffolds with ZnO nanoparticles was high throughout the scaffold up to 2 wt% with statistical significance (P ≤ 0.05), whereas the cell density in control and at higher concentrations of ZnO nanoparticles, above 2 wt% was rather less. Compared to bare PCL scaffolds, 1 wt% ZnO nanoparticle containing scaffolds had shown higher cell density in each point during the incubation with very high to extreme statistical significance (P value between 0.05 to 0.0004).
| Sample | Cell density (cells per mm2) | ||||
|---|---|---|---|---|---|
| After 24 h | After 48 h | After 72 h | After 96 h | After 120 h | |
| PCL alone | 43 ± 7 | 167 ± 8 | 182 ± 10 | 211 ± 7 | 222 ± 13 |
| PCL/ZnO-0.5 wt% | 48 ± 8 | 193 ± 5 | 213 ± 7 | 243 ± 6 | 253 ± 7 |
| PCL/ZnO-1 wt% | 56 ± 5 | 214 ± 4 | 246 ± 6 | 281 ± 9 | 313 ± 7 |
| PCL/ZnO-2 wt% | 51 ± 4 | 206 ± 5 | 226 ± 8 | 258 ± 4 | 267 ± 5 |
| PCL/ZnO-4 wt% | 16 ± 8 | 86 ± 6 | 106 ± 5 | 117 ± 4 | 128 ± 8 |
| PCL/ZnO-6 wt% | 7 ± 3 | 45 ± 9 | 76 ± 4 | 86 ± 7 | 92 ± 3 |
3.4. CAM assay
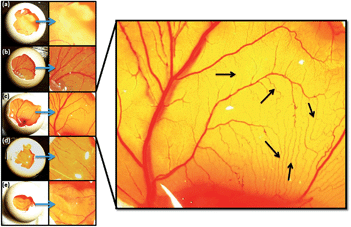
The chicken chorioallantoic membrane (CAM) assay is a standard assay used to evaluate the angiogenic potential of biomaterials. CAM assay was carried out in order to investigate the ability of ZnO nanoparticles present in the PCL scaffolds to induce angiogenesis. Fig. 4(a) and (e) indicates the results of CAM assay where the neat PCL scaffolds without nanoparticles (control: Fig. 4(a)), PCL scaffolds containing ZnO nanoparticles at 0.5 wt% (Fig. 4(b)), 1 wt% (Fig. 4(c)), 2 wt% (Fig. 4(d)) and 4 wt% (Fig. 4(e)) concentrations were used. PCL scaffolds with ZnO nanoparticles significantly increased the formation of matured vascular sprouting with increasing concentration up to 1 wt%. In the case of PCL scaffolds containing 1 wt% ZnO nanoparticles, large number of matured blood vessels with highly branched capillary network could be observed. While higher concentrations of ZnO nanoparticles did not considerably increase the angiogenesis.To quantify the ability of PCL scaffolds containing ZnO nanoparticles to promote angiogenesis, number of branching points were counted and given in Table 2. The scaffolds containing 1 wt% ZnO nanoparticles showed very statistically significant proangiogenic property with branching points of 123 ± 16 compared to the bare PCL scaffold which produced 68 ± 13 branching points only (P = 0.0099). The PCL scaffolds containing 0.5 wt% ZnO nanoparticles also showed a little proangiogenic property than the bare PCL scaffolds (P = 0.5885) but was much less than that containing 1 wt% ZnO nanoparticles. Compared to the PCL scaffolds containing 1 wt% ZnO nanoparticles, both the scaffold containing 2 wt% (P = 0.0462) and 4 wt% (P = 0.0103) ZnO nanoparticles showed less proangiogenic activity with statistical significance. There was no statistical significance between the proangiogenic property of bare PCL scaffolds and those containing 2 wt% (P = 0.9152) and 4 wt% (P = 0.6154). Considering the results of CAM assay, it is clear that PCL scaffolds with 1 wt% of ZnO nanoparticles are proangiogenic.
| Sample | No. of branching points |
|---|---|
| PCL alone | 68 ± 13 |
| PCL/ZnO-0.5 wt% | 74 ± 12 |
| PCL/ZnO-1 wt% | 123 ± 16 |
| PCL/ZnO-2 wt% | 91 ± 11 |
| PCL/ZnO-4 wt% | 67 ± 14 |
3.5. In vivo angiogenesis
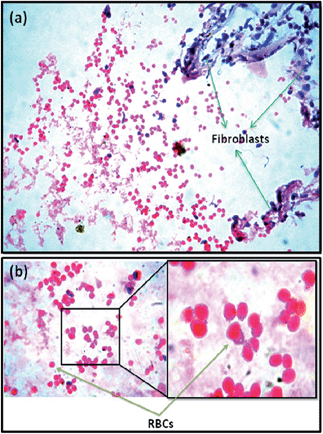
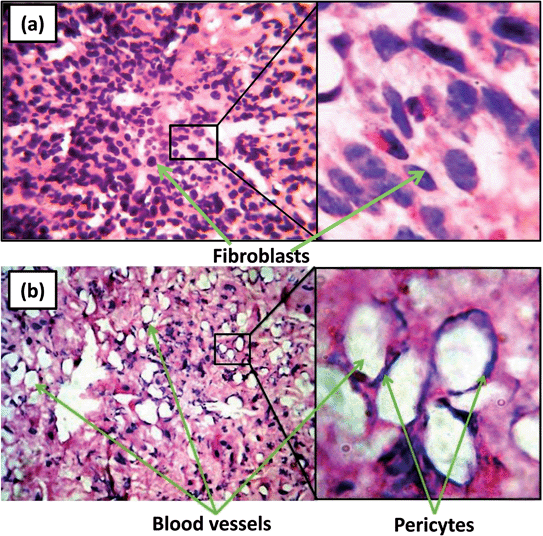
Based on the results of CAM assay and in vitro cell culture studiers, 1 wt% ZnO nanoparticles incorporated PCL scaffolds were selected for animal implantation studies to evaluate the in vivo angiogenesis. The neat PCL scaffolds as well as that containing 1 wt% ZnO particles were subcutaneously implanted in guinea pigs and recovered for histological evaluations. At the 5th day of implantation itself, initiation of angiogenesis could be observed in PCL scaffolds containing 1 wt% ZnO nanoparticles (Fig. 5). Large number of red blood cells (RBCs) were migrated to the membrane and arranged in a circular fashion which is considered as the first event of vascular sprouting. Further we could observe large number of fibroblasts migrating from the sides of the scaffolds to the interior (Fig. 5(a)). We did not observe such a vascular sprouting in the case of neat scaffolds.Fig. 6 gives the direct evidence for the formation of blood vessel through the ZnO nanoparticle containing membrane. From the figure, it can be observed that two large blood vessels were passing through the subcutaneously implanted membrane at 20th day of implantation. Transverse section of this membrane shows the presence of extensive vascular network throughout it (Fig. 7(b)). Blood capillaries are formed of endothelial cells that form the inner lining of the wall with a surrounding basal lamina and pericytes that extend long cytoplasmic processes over the surface of the vascular tube. The enlarged view clearly shows the presence of pericytes around the blood vessels which is an indication of the mature vessels. Neat PCL scaffolds did not show any sign of angiogenesis through them even after 20 days of implantation (Fig. 7(a)).
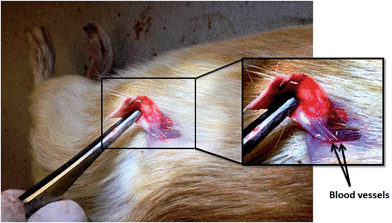 | ||
| Fig. 6 Matured blood vessel through the implanted PCL scaffolds containing 1 wt% ZnO nanoparticles after 20 days of subcutaneous implantation. | ||
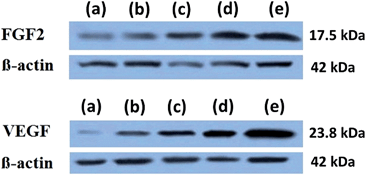
3.6. VEGF-A and FGF2 expression
Western blots were used to determine the effect of ZnO nanoparticles on the expression of FGF2 and VEGF-A and are given in Fig. 8. When ZnO nanoparticles were incorporated into the PCL scaffolds, HDFa cells grown in the scaffolds have shown an increase in the level of expression of both VEGF-A and FGF2. Protein isolated from the cells cultured on neat PCL scaffolds showed lowest level of expression of both FGF2 and VEGF-A. As the concentration of ZnO nanoparticles increased to 4 wt% in the PCL membrane, expression of both FGF2 and VEGF-A increased.4 Discussion
Electrospinning of polycaprolactone,41 its blends,42 nanocomposites43 and their use in tissue engineering as biodegradable scaffolds is already reported. We have successfully fabricated PCL scaffolds incorporated with ZnO nanoparticles by electrospinning technique. Our FESEM and EDAX results confirmed the presence of ZnO nanoparticles in the PCL fibres. Photocatalytic production of reactive oxygen species (ROS) like H2O2 by ZnO nanoparticles is well established by previous works.44 Further the cells themselves produce reactive oxygen species in response to the stress induced by ZnO nanoparticles.45 In vitro HDFa cell proliferation study showed that presence of ZnO nanoparticles enhanced the cell attachment on the scaffold. 1 wt% ZnO nanoparticle incorporated scaffold was the best material in terms of the cell attachment. The enhanced cell attachment might be due to the expression of growth factors like FGF mediated by the ROS generated by ZnO nanoparticles. Higher concentration of ROS is toxic to the biological system and this might be the reason for the decreased cell attachment at higher concentration of ZnO nanoparticles in the scaffold.46 This is in agreement with our previous studies of PCL membranes containing ZnO nanoparticles on goat fibroblast cells26 and in vivo implantation studies in guinea pigs.28Both CAM assay and in vivo implantation study had shown that ZnO nanoparticle incorporated PCL scaffolds were proangiogenic. Formation of ROS, especially H2O2, might be the plausible mechanism for ZnO nanoparticle induced angiogenesis. Ayan et al. demonstrated that ZnO nanoflowers can induce angiogenesis, here also the ZnO nanoparticles present in the PCL scaffolds induced proliferation and migration of endothelial cells and led to the formation of new blood vessels.29 Patra et al. also suggested ROS driven angiogenesis by europium hydroxide [EuIII(OH)3] nanorods.31,33 The decrease in angiogenic property of the scaffolds at higher concentrations of ZnO nanoparticles (2 and 4 wt%) might be due to the higher level of ROS species which might be negatively affected the angiogenic factors or induced cell damage.46 It is well established that ROS play an important role in angiogenesis by stimulating the key steps of cell proliferation, migration and tube formation.21,47 Among all the biologically important ROS, H2O2 is considered as the most important one that is involved in redox signaling events during angiogenesis.19 Thus, generation of ROS, especially H2O2, by ZnO nanoparticles might be the plausible mechanism for in vitro and in vivo angiogenesis in the PCL/ZnO membranes.
To demonstrate the molecular mechanism of enhanced angiogenesis and cell proliferation shown by the ZnO nanoparticles incorporated scaffolds, western blotting analysis has been carried out. Upregulation of FGF2 and VEGF was observed as a function of ZnO nanoparticle concentration in the PCL scaffolds. FGF was found to be important for new blood vessel growth at the site of an excision wound and have been shown to stimulate angiogenesis in various assays.48 FGF-2 is a very potent inducer of angiogenesis. In a preclinical model of microvascular endothelial cells grown on a 3-dimensional collagen gel, VEGF-A and FGF-2 were able to induce cells to invade the underlying matrix to form capillary-like tubules.49
Vascular endothelial growth factor (VEGF) is one of the most important angiogenic mediators characterized to date.50 Level of VEGF in the cells cultured on neat PCL scaffolds was very less. Upregulation of VEGF-A in presence of ZnO nanoparticles might be due to the generation of H2O2 by ZnO nanoparticles. Previous report suggests that H2O2 induces VEGF expression in human keratinocytes,16 retinal pigment epithelial cells,50 endothelial cells,51 murine fibroblasts50 and macrophages.52 Hydrogen peroxide (H2O2) enhanced the wound healing in mice by the expression of VEGF.16
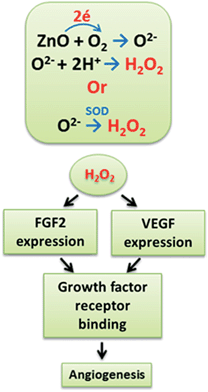
A schematic representation of anticipated mechanism of action of ZnO nanoparticles in the PCL scaffolds is given in Fig. 9. We hypothesize that the ZnO nanoparticles in PCL matrix can play an important role in the generation of ROS, especially H2O2. Multiple mechanisms may exist in the generation of H2O2 in the cells; direct production O2− radicals and the reaction with water may be one mechanism.27,28 Another plausible mechanism may be the conversion of produced super oxides into H2O2 by superoxide dismutase enzyme inside the cells.53 Generated H2O2 will enhance the expression of FGF and VEGF by the cells. These upregulated growth factors will bind to more receptors to them and activate downstream effectors. This will trigger endothelial cell proliferation, migration and tube formation through the polymeric scaffolds. Thus, generation of ROS, especially H2O2, by ZnO nanoparticles might be the plausible mechanism for in vitro and in vivo angiogenesis in the PCL/ZnO scaffolds. The fabricated scaffolds containing ZnO nanoparticles can be used for various tissue engineering applications like skin tissue engineering, soft tissue engineering and bone regeneration where angiogenesis is crucial.
5 Conclusions
In this study, electrospun polycaprolactone (PCL) scaffolds incorporated with zinc oxide nanoparticles were successfully fabricated and characterized. The fabricated membranes were highly porous with randomly oriented fibers. The EDX analysis confirmed the presence of ZnO nanoparticles in the electrospun scaffolds. The CAM assay was carried out in order to investigate the ability of ZnO nanoparticles present in the PCL membrane to induce angiogenesis. In the case of PCL membranes containing 1 wt% ZnO nanoparticles, large number of matured blood vessels with highly branched capillary network were observed, however higher concentrations of ZnO nanoparticles did not considerably increase the angiogenesis. In vivo implantation study in guinea pigs confirmed the formation of well-developed vasculature through the scaffold. Protein expression study showed that the enhanced angiogenic property of the PCL scaffolds containing ZnO nanoparticles is due to the upregulation of key angiogenic factors, FGF and VEGF. Considering the results of this study, it is clear that PCL membranes containing ZnO nanoparticles are proangiogenic. Thus, the fabricated material can be used as a successful tissue engineering scaffold material to induce angiogenesis through the scaffold which will enhance the integration of scaffold and the host tissue.Acknowledgements
The authors acknowledge Department of Biotechnology (DBT), Government of India, New Delhi for the financial support through MSUB IPLSARE program. Funding from DST-Nanomission, UGC SAP, DST-FIST, SAARD-KSCSTE are also gratefully acknowledged.References
- A. B. Ennett and J. M. David, Expert Opin. Biol. Ther., 2002, 2, 805–818 CrossRef CAS PubMed.
- J. Rouwkema, C. R. Nicolas and A. v. B. Clemens, Trends Biotechnol., 2008, 26, 434–441 CrossRef CAS PubMed.
- J. Folkman and Y. Shing, J. Biol. Chem., 1992, 267, 10931–10934 CAS.
- T. O. Daniel and D. Abrahamson, Annu. Rev. Physiol., 2000, 62, 649–671 CrossRef CAS PubMed.
- M. A. Nugent and R. V. Iozzo, Int. J. Biochem. Cell Biol., 2000, 32, 115–120 CrossRef CAS PubMed.
- T. V. Petrova, T. Makinen and K. Alitalo, Exp. Cell Res., 1999, 253, 117–130 CrossRef CAS PubMed.
- S. Davis, T. H. Aldrich and P. F. Jones, et al., Cell, 1996, 87, 1161–1169 CrossRef CAS PubMed.
- B. P. Eliceiri and D. A. Cheresh, J. Clin. Invest., 1999, 103, 1227–1230 CrossRef CAS PubMed.
- T. Tokunaga, M. Nakamura and Y. Oshika, et al., Br. J. Cancer, 1999, 79(2), 354–359 CrossRef CAS PubMed.
- M. S. O'Reilly, L. Holmgren and Y. Shing, et al., Cell, 1994, 79, 315–328 CrossRef.
- M. S. O'Reilly, T. Boehm and Y. Shing, et al., Cell, 1997, 8(8), 277–285 CrossRef.
- H. Madry, A. Rey-Rico and J. K. Venkatesan, et al., Tissue Eng., Part B, 2013, 20(2), 106–125 CrossRef PubMed.
- S. Nillesen, P. J. Geutjes and R. Wismans, et al., Biomaterials, 2007, 28(6), 1123–1131 CrossRef CAS PubMed.
- T. P. Richardson, M. C. Peters and A. B. Ennett, et al., Nat. Biotechnol., 2001, 19(11), 1029–1034 CrossRef CAS PubMed.
- G. H. Kim and H. Yoon, Appl. Phys. A: Mater. Sci. Process., 2008, 90(3), 389–394 CrossRef CAS.
- C. K. Sen, S. Khanna and B. M. Babior, et al., J. Biol. Chem., 2002, 277(36), 33284–33290 CrossRef CAS PubMed.
- S. Roy, S. Khanna and K. Nallu, et al., Mol. Ther., 2006, 13(1), 211–220 CrossRef CAS PubMed.
- Y. Huo, W. Y. Qiu and Q. Pan, et al., Exp. Eye Res., 2009, 89(6), 876–886 CrossRef CAS PubMed.
- P. I. Lelkes, K. L. Hahn and D. A. Sukovich, et al., On the possible role of reactive oxygen species in angiogenesis, in Oxygen Transport to Tissue XX, Springer, USA, 1998, pp. 295–310 Search PubMed.
- S. G. Rhee, Science, 2006, 577(312), 1882–1883 CrossRef PubMed.
- C. Xia, Q. Meng and L. Z. Liu, et al., Cancer Res., 2007, 67(22), 10823–10830 CrossRef CAS PubMed.
- M. Ushio-Fukai, FASEB J., 2006, 20, A21 Search PubMed.
- R. Dardik, J. Loscalzo, R. Eskaraev and A. Inbal, Arterioscler., Thromb., Vasc. Biol., 2005, 25(3), 526–532 CrossRef CAS PubMed.
- P. A. Pechan, K. Chowdhury and W. Seifert, Neuroreport, 1992, 3(6), 469–472 CrossRef CAS PubMed.
- M. Wada, C. M. Gelfman and H. Matsunaga, et al., Curr. Eye Res., 2001, 23(3), 226–231 CrossRef CAS PubMed.
- R. Augustine, H. N. Malik and D. K. Singhal, et al., J. Polym. Res., 2014, 21(3), 1–17 CAS.
- D. J. M. Bevan and J. S. Anderson, Discuss. Faraday Soc., 1950, 8, 238–246 RSC.
- R. Augustine, E. A. Dominic and I. Reju, et al., RSC Adv., 2014, 4, 24777–24785 RSC.
- K. B. Ayan, V. Vimal and M. Sudip, et al., Zinc oxide nanoflowers make new blood vessels, Nanoscale, 2012, 4, 7861–7869 RSC.
- C. R. Patra, R. Bhattacharya and S. Patra, et al., Clin. Chem., 2007, 53(11), 2029–2031 CAS.
- C. R. Patra, R. Bhattacharya and S. Patra, et al., Adv. Mater., 2008, 20(4), 753–756 CrossRef CAS.
- C. R. Patra, S. S. A. Moneim and E. Wang, et. al., Toxicol. Appl. Pharmacol., 2009, 240(1), 88–98 CrossRef CAS PubMed.
- C. R. Patra, J. H. Kim and K. Pramanik, et al., Nano Lett., 2011, 11(11), 4932–4938 CrossRef CAS PubMed.
- D. W. Hutmacher, J. T. Schantz and C. X. F. Lam, et al., J. Tissue Eng. Regener. Med., 2007, 1(4), 245–260 CrossRef CAS PubMed.
- L. Calandrelli, A. Calarco and P. Laurienzo, et al., Biomacromolecules, 2008, 9(6), 1527–1534 CrossRef CAS PubMed.
- B. M. Min, L. Jeong and Y. S. Nam, et al., Int. J. Biol. Macromol., 2004, 34(5), 223–230 CrossRef PubMed.
- J. J. Kim, R. K. Singh and S. J. Seo, et al., RSC Adv., 2014, 4(33), 17325–17336 RSC.
- S. Kumar, S. Bose and K. Chatterjee, RSC Adv., 2014, 4(37), 19086–19098 RSC.
- R. Kang, Y. Luo and L. Zou, et al., RSC Adv., 2014, 4(11), 5734–5739 RSC.
- S. Salehi, T. Bahners and J. S. Gutmann, et al., RSC Adv., 2014, 4(33), 16951–16957 RSC.
- H. Yoshimoto, Y. M. Shin and H. Terai, et al., Biomaterials, 2003, 24(12), 2077–2082 CrossRef CAS PubMed.
- L. Ghasemi-Mobarakeh, M. P. Prabhakaran and M. Morshed, et al., Biomaterials, 2008, 29(34), 4532–4539 CrossRef CAS PubMed.
- P. Wutticharoenmongkol, N. Sanchavanakit and P. Pavasant, et al., Macromol. Biosci., 2006, 6(1), 70–77 CrossRef CAS PubMed.
- A. J. Hoffman, R. C. Elizabeth and R. H. Michael, Environ. Sci. Technol., 1994, 28(5), 776–785 CrossRef CAS PubMed.
- D. Guo, H. Bi and B. Liu, et al., Toxicol. In Vitro, 2013, 27, 731–738 CrossRef CAS PubMed.
- B. Halliwell, Reactive oxygen species and the central nervous system in Free Radicals in the Brain, Springer Berlin Heidelberg, 1992, pp. 21–40 Search PubMed.
- M. Ushio-Fukai, Cardiovasc. Res., 2006, 71(2), 226–235 CrossRef CAS PubMed.
- D. G. Greenhalgh, K. H. Sprugel and M. J. Murray, et al., Am. J. Pathol., 1990, 136, 1235–1246 CAS.
- M. S. Pepper, N. Ferrara and L. Orci, et al., Biochem. Biophys. Res. Commun., 1992, 189(2), 824–831 CrossRef CAS PubMed.
- J. Cisowski, A. Loboda and A. Józkowicz, et al., Biochem. Biophys. Res. Commun., 2005, 326(3), 670–676 CrossRef CAS PubMed.
- A. Sanchez, D. Tripathy and J. Luo, et al., J. Alzheimer's Dis., 2013, 34(1), 281–291 CAS.
- M. Cho, T. K. Hunt and M. Z. Hussain, Am. J. Physiol.: Heart Circ. Physiol., 2001, 280(5), H2357–H2363 CAS.
- I. Fridovich, Annu. Rev. Biochem., 1975, 44(1), 147–159 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |