Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Combined heterogeneous bio- and chemo-catalysis for dynamic kinetic resolution of (rac)-benzoin†
R.
Nieguth
a,
J.
ten Dam
b,
A.
Petrenz
c,
A.
Ramanathan
bd,
U.
Hanefeld
b and
M. B.
Ansorge-Schumacher
*ac
aLehrstuhl für Technische Chemie/Enzymtechnologie, Institut für Chemie, Technische Universität Berlin, 10623 Berlin, Germany
bGebouw voor Scheikunde, Biokatalyse, Afdeling Biotechnologie, Technische Universiteit Delft, Julianalaan 136, 2628 BL, Delft, The Netherlands
cProfessur für Molekulare Biotechnologie, Institut für Mikrobiologie, Technische Universität Dresden, 01062 Dresden, Germany. E-mail: marion.ansorge@tu-dresden.de; Fax: +49 351 46339520; Tel: +49 351 46339518
dCenter for Environmentally Beneficial Catalysis, The University of Kansas, Lawrence, Kansas 66047, USA
First published on 15th September 2014
Abstract
Dynamic kinetic resolution (DKR) of racemic starting material is a promising route to optically pure (S)-benzoin (2-hydroxy-1,2-di(phenyl)ethanone) and various symmetrical and unsymmetrical derivatives thereof. Here, this route was advanced towards technical scale synthesis using the basic (rac)-benzoin as model system. The reaction employed stereoselective transesterification of (S)-benzoin with lipase TL® from Pseudomonas stutzeri and racemization of (R)-benzoin with Metal-TUD-1, a metal-associated acidic meso-porous silicate, in pure organic solvent. Enzyme performance was improved by immobilization on Accurel MP1001 (yielding Acc-LipTL), and Zr-TUD-1 (Si/Zr = 25) was identified as most effective racemization catalyst. Compatibility in solvent and temperature dependency enabled performance in only one pot. DKR in toluene at 50 °C yielded conversions above 98% and an ee of >97% in only five hours. Stability of Acc-LipTL was further improved with polyethylene imine and the reaction system was then reused in five cycles, retaining a conversion of >99% and a product-ee of >98%. On a semi-preparative scale, the isolated yield of enantiopure (S)-benzoin butyrate was >98%. Thus, the system provides a good basis for synthesis of enantiopure benzoin, and potentially a broader range of aromatic α-hydroxy ketones.
Introduction
Enantiomerically pure acyloins (α-hydroxy ketones) provide important structural motifs for a number of fine chemicals and pharmaceuticals.1–3 Hence, various synthetic routes, chemo- or biocatalytic, have been described to date.2 They considerably differ in selectivity and yield, as well as in the accessible range of concrete structures (i.e. aliphatic, heterocyclic, aromatic, symmetrical, unsymmetrical, R-/S-stereochemistry, etc.) giving a portfolio of potentially interesting methods.Dynamic kinetic resolution (DKR) of racemic starting material by combination of a biocatalysed kinetic resolution (KR) with in situ racemisation (RAC) of the undesired substrate enantiomer (Scheme 1) has recently been described as a promising route to optically pure (S)-benzoin (2-hydroxy-1,2-di(phenyl)ethanone) and various symmetrical and unsymmetrical derivatives of this basic aromatic α-hydroxy ketone.3–5
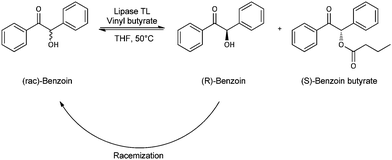 | ||
| Scheme 1 Dynamic kinetic resolution of benzoin using vinyl butyrate as acyl donor and Lipase TL® as catalyst. | ||
The method involves stereoselective transesterification of (rac)-benzoin with a lipase from Pseudomonas stutzeri (commercialised by Meito Sangyo Co., Japan, under the trade name of Lipase TL®, and immobilised through entrapment of the crude enzyme powder in a silicone matrix) and RAC with a homogeneous ruthenium based transition metal catalyst (Shvo's catalyst) and achieves substrate conversions above 90%. As product, (S)-configured butyrate esters with an enantiomeric excess above 99% are obtained, from which enantiopure (S)-benzoin derivatives can easily be released through hydrolysis (e.g. using Lipase TL® under suitably adapted conditions). Despite this encouraging performance, however, the approach has not been transferred to synthetic application on a larger scale to date due to several unfavourable characteristics: (1) reproducibility of catalytic efficiency between separate reaction batches is low. Most probably this is connected to the preparation of the biocatalyst which must lead to an uneven and uncontrollable distribution of the lipase within the particles and hence to widely varying catalytic performance. Additionally, sensitivity of the ruthenium catalyst for traces of molecular oxygen in the reaction set-up, and interference of acetaldehyde released from vinyl butyrate (i.e. the optimal acyl donor for benzoin esterification4) with the ruthenium catalyst6 could be an issue. (2) The ruthenium catalyst cannot be recycled, which increases both costs for reaction and costs for product workup. (3) Reaction conditions required for optimal performance and stability of bio- and chemo-catalyst (e.g. temperature) are hardly compatible resulting in low total turnover numbers and consequently large catalyst consumption. However, this specific item could recently be overcome by introduction of a novel ruthenium complex for racemization.7
This paper describes the advancement of DKR for technical scale hydroxy ketone synthesis with regard to reproducibility, catalyst recyclability and performance in one pot using DKR of (rac)-benzoin as model system. The study involved systematic development of a controllable and reproducible protocol for Lipase TL® immobilisation and introduction of a heterogeneous metal catalyst for racemization. Kinetic resolution and racemization of benzoin with these catalysts were characterised both separately and in a one-pot DKR. Optimisation was performed with regard to reaction medium and temperature. The synthetic potential of the approach was demonstrated on a semi-preparative scale.
Results and discussion
Immobilisation of lipase TL®
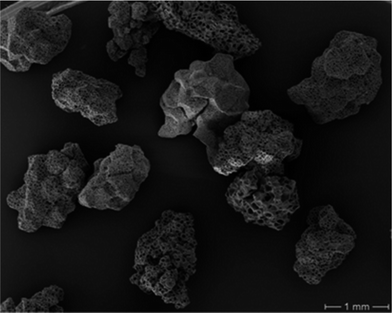
For immobilisation of Lipase TL® (further annotated as LipTL) adsorption to the porous polypropylene resin Accurel MP1001 was selected. Leaching of enzyme during reaction must not be expected due to its insolubility in hydrophobic solvents making covalent attachment unnecessary.Accurel is a highly hydrophobic material, for which beneficial effects on the catalytic behaviour of bound enzymes has been reported before.8 The obtained material had a size distribution of 400–1000 μm in diameter (Fig. 1) and a specific surface of 35 m2 (according to the BET method). The surface topology of individual particles, especially regarding pore size, was rather diverse. It can be assumed, however, that all microscopically visible pores are macroporous (i.e. displaying a diameter >500 nm) and are thus at least one to two orders of magnitudes larger than LipTL. Size differences should therefore be of only minor importance to the uptake of this enzyme.
Enzyme immobilisation by adsorption is a spontaneous process running into a distinct thermodynamic equilibrium of bound and unbound protein. The position of equilibrium is influenced by many factors including carrier constitution and preparation conditions. A key role is played by the protein concentration in the immobilisation set-up.9 The amount of protein bound to the carrier is usually proportional to the available surface of the carrier until the binding capacity is exceeded, the protein aggregates, or multilayers are formed. Accordingly, optimum conditions for adsorption of LipTL on Accurel MP1001 were determined by introducing different concentrations of the crude preparation into the immobilisation process. Success was evaluated with regard to the specific catalytic activity of the resulting immobilisates in the transesterification of benzoin with vinyl butyrate using tetrahydrofuran (THF) as solvent.4,10 The immobilised preparation is further denoted as Acc-LipTL.
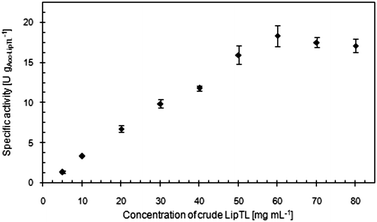
The specific catalytic activity of Acc-LipTL increased with increasing concentrations of LipTL in the immobilisation process up to a steady value of 18.4 U gAcc-LipTL−1 correlating to a powder concentration of and above 60 mgLipTL mLimmobilisation medium−1 (Fig. 2). Loading at this concentration was determined as 15.4 mgProtein gAccurel−1 and accordingly, the specific catalytic activity related to the protein content was 1192 U gProtein−1, which is by a factor of three higher than the specific activity of the crude LipTL preparation (i.e. 350 U gProtein−1).
An increase in the specific catalytic activity of LipTL through immobilisation (by factor 2.2) had also been observed by Hoyos et al.5 As an explanation the enhanced transfer of the hydrophobic substrates and products through the also hydrophobic silicone matrix, and a stabilisation of the catalytic active conformation of LipTL (generally recognised as hyperactivation or interfacial activation) were suggested. This active conformation usually describes a state where an amphipathic peptide chain (lid) at the active site of the enzyme is open due to the interaction of its hydrophobic part with a hydrophobic interface or surface,11 and it has only lately been reported that LipTL might contain even two such lids.12 Both explanations could equally apply to the behaviour of LipTL on Accurel MP1001 due to the distinct hydrophobicity of this material. In addition – or alternatively – a selective binding of LipTL to the carrier could occur which would result in a purification of the enzyme. According to Maraite et al.,12 commercial LipTL contains an assortment of side proteins that might (at least partly) have a significantly lower affinity to the hydrophobic support than the lipase itself. Even simpler, the activity increase on Accurel MP1001 could be connected with the more even distribution of the enzyme, and therefore better accessibility, on the carrier compared to suspended (and usually aggregated) solid enzyme in pure organic solvents. Overall, the results strongly support the suitability of Accurel MP1001 as a carrier for LipTL.
On the assumption that the observed maximum specific activity of Acc-LipTL at a concentration of 60 mgLipTL mLimmobilisation medium−1 correlated to the arrival at the maximum binding capacity and might therefore prohibit detection of further effects on activity, further experiments on the optimisation of the immobilisation process were performed at a constant concentration of 30 mgLipTL mLimmobilisation medium−1. Buffer (KPi) concentration and pH were varied between 10 mmol L−1 and 400 mmol L−1 and around the isoelectric point of LipTL (i.e. 6.6) to pH 6.2, pH 6.6 and pH 7.0, respectively.
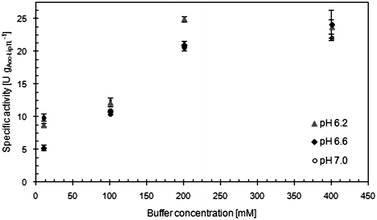
Optimum conditions with regard to the specific catalytic activity of Acc-LipTL were found at a buffer concentration of 200 mmol L−1 and pH 6.2, with the buffer concentration as the decisive parameter (Fig. 3). The maximum specific activity was 24.7 U gAcc-LipTL−1. At a loading of 17.8 mgProtein gAccurel−1, this corresponded to a protein related specific activity of 1.4 U gProtein−1, which was an increase by factor 4.1 compared to crude LipTL and was almost two-fold better than the effect of silicone entrapment as described by Hoyos et al.5
The findings on buffer and pH effects are in good agreement with the assumption that the adsorptive binding of proteins to the nonionic Accurel MP1001 is predominantly promoted by hydrophobic interactions: changes of pH have only minor effects on such binding forces, while phosphate anions comprising the buffer are kosmotropic in nature and are therefore strengthening them. The same effects are exploited in hydrophobic interaction chromatography, where high ionic strengths are used to selectively bind hydrophobic proteins.13
Transfer of these optimum conditions to LipTL immobilisation at an initial powder concentration of 60 mgLipTL mLimmobilisation medium−1 resulted in a minor improvement of the specific catalytic activity to 28.2 U gAcc-LipTL−1, which was in perfect agreement with the initial assumption on already saturated loading at this protein concentration. As the poor additional benefit did not justify consumption of the double amount of the valuable enzyme, a LipTL concentration of 30 mgLipTL mLimmobilisation medium−1 was established in the final immobilisation protocol.
KR of (rac)-benzoin
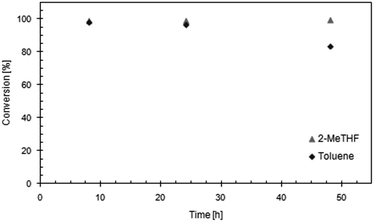
Acc-LipTL was subjected to KR of (rac)-benzoin using dried THF, 2-MeTHF and toluene, respectively, as solvents. The selection was based on the reported suitability of THF and its environmentally benign derivative 2-methyl tetrahydrofuran (2-MeTHF) for kinetic resolution of benzoin,4,10,14 and of toluene for racemization.15 At room temperature (21.5 °C), Acc-LipTL displayed good activity in all three solvents (Table 1, column 3). The time to reach maximum conversion (i.e. 50%), however, was considerably shorter in 2-MeTHF and toluene (2.5 h) than in THF (>4 h) (Table 1, column 4). The half-life of Acc-LipTL under process conditions (calculated from the decrease of conversion after reaching steady-state in a continuous reaction set-up) was about 1.6-fold and 2.9-fold higher in 2-MeTHF than in toluene and THF, respectively.Similar results were obtained at 50 °C, which is the temperature preferable for running the racemization catalyst. Interestingly, the specific activity of Acc-LipTL in toluene now exceeded the activity in 2-Me-THF, while the time to obtain full conversion was still similar. The time to reach full conversion in THF changed only slightly compared to the reaction at room temperature. Both findings can easily be explained by a lower stability of Acc-LipTL in toluene and THF, respectively, than in 2-MeTHF confirming the observations on process stability described above. Notably, in both 2-Me-THF and toluene KR can obviously be run efficiently at 50 °C.
Racemization of (R)-benzoin
Apart from the homogeneous Shvo's catalyst, several heterogeneous catalysts display activity on secondary alcohols including transition metals supported on carbon, hydroxyapatite or alumina, acidic resins, vanadium catalysts and zeolites.16,17 As demonstrated in earlier studies, commercial ion-exchangers exert a detrimental effect on enzyme activity,18 and are therefore not suitable for a one pot DKR as targeted in this study. Heterogeneous (semi-) precious metal catalysts, on the other hand, usually suffer from high costs and leaching of the active species, while Zeolites have only small pore sizes limiting their application to small substrates. Both disadvantages, however, can be overcome by use of mesoporous material: framework incorporation of active metals suppresses leaching and large pores allow full accessibility even for large substrates.19,20 TUD-1 is a mesoporous material with a sponge-like structure and three-dimensional pores.21 Its environmentally benign synthesis allows for the straightforward introduction of a large range of metals into the framework. High stability and recyclability has been demonstrated in many different reactions, in particular in the Meerwein–Ponndorf–Verley reduction and the Oppenauer oxidation.19,21–23 Moreover, its induced acidic sites make it ideal for the generation of carbenium ions, intermediates in the racemization of benzylic alcohols, while side reactions often observed with commercially available acid resins do not occur. Accordingly, TUD-1 catalysts were tested for racemization of (R)-benzoin. As incorporated metals, aluminium,24,25 zirconium,15,26 and tungsten,27 respectively, were involved.Based on the findings on KR performance with Acc-LipTL (see previous section) and on the study of Ramanathan et al.,15 toluene was chosen as the solvent for racemization. The racemization success was measured in terms of enantiomeric excess (ee), for which in this case a value as small as possible was targeted. Results are summarized in Table 2.
| Entry | Catalyst | Time [h] | ee [%] |
|---|---|---|---|
| 1 | TUD-1 | 20 | 93.8 |
| 2 | Zr-TUD-1 (Si/Zr = 25) | 20 | 1.3 |
| 3 | Al-TUD-1 (Si/Al = 4) | 20 | 0.2 |
| 4 | Al-TUD-1 (Si/Al = 25) | 20 | 23.2 |
| 5 | W-TUD-1 (Si/W = 10) | 20 | 65.7 |
| 6 | W-TUD-1 (Si/W = 20) | 20 | 43.9 |
| 7 | W-TUD-1 (Si/W = 30) | 20 | 63.9 |
| 8 | W-TUD-1 (Si/W = 40) | 20 | 73.7 |
| 9 | W-TUD-1 (Si/W = 50) | 20 | 71.7 |
| 10 | Zr-TUD-1 (Si/Zr = 25) | 4 | 4.4 |
| 11 | Al-TUD-1 (Si/Al = 4) | 4 | 42.8 |
It was observed that purely siliceous TUD-1 (i.e. without any metal incorporated) was already able to catalyse the racemization of (R)-benzoin, although not very efficiently. An ee of 93.8% was obtained after 20 h at 50 °C. Under the same conditions, introduction of tungsten to TUD-1 (W-TUD-1) yielded a maximum ee of 43.9% at a ratio of Si/W = 20. Increases in the metal content initially enhanced the reaction rate, while after surpassing a maximum racemization activity, high loadings of tungsten were less efficient. As tungsten provides the acidic reaction site for racemization, this behaviour is a reaction to the actual availability of reactive centers. It increases with incorporation of tungsten until the formation of crystalline tungsten oxide species with a reduced surface area and therefore lower accessibility of the acidic metal occurs.
Excellent racemization results were obtained with aluminum and zirconium as incorporated metals. After 20 h at 50 °C both Zr-TUD-1 (Si/Zr = 25) and Al-TUD-1 (Si/Al = 4) had completely racemised (R)-benzoin. After a reaction time of only four hours which is in the time range of KR with Acc-LipTL in toluene, (see previous section) Zr-TUD-1 (Si/Zr = 25) reduced the ee to 4.4%, while Al-TUD-1 (Si/Al = 4) managed a final ee of 42.8%. Thus, Zr-TUD-1 demonstrated a superior racemization activity, which is most likely due to the purely Lewis acidic nature of zirconium in the framework of TUD-1,19 contrasting the mixed Lewis and Brønsted acid character imparted by tungsten and aluminum.15,22,25 Importantly, Zr-TUD-1 showed no racemization activity with (R)-benzoin butyrate (within two hours) even at high temperature (70 °C) and thus ensures that the enantiopurity of the KR product cannot be affected.
For investigation of temperature effects on the racemization efficiency both Zr-TUD-1 (Si/Zr = 25) and Al-TUD-1 (Si/Al = 4) were subjected to higher reaction temperatures (50, 70 and 90 °C). As expected from the Arrhenius relation, the racemization rate increased with increasing temperature in both cases (Fig. 4). With Zr-TUD-1 (Si/Zr = 25) complete racemization was achieved within 90 minutes at 70 °C and only 30 minutes at 90 °C. With Al-TUD-1 (Si/Al = 4) considerably longer reaction times were required. The minimum was 90 minutes at a temperature of 90 °C. Notably, only small amounts of benzil, the dicarbonyl analogue of benzoin, were detected in all set-ups indicating that dehydrogenation, which had been described in connection with acidic racemization of hydroxyl compounds before,17,18,28 plays a minor role in the racemization of benzoin with the selected TUD-1 catalysts.
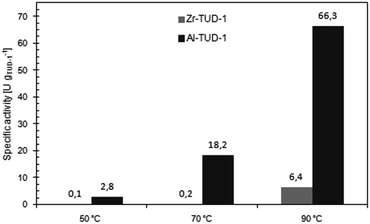
The tendency of Zr-TUD-1 and Al-TUD-1 to catalyze unspecific transesterification of benzoin in the final reaction mixture as a likely side reaction17,18,28 was investigated by incubation of a mixture of catalyst, (rac)-benzoin and vinyl butyrate in toluene at different temperatures. Under all conditions strong transesterification activity was observed with Al-TUD-1 (Fig. 5), yielding benzoin ester amounts comparable to the enzymatic reaction. Due to the fully racemic nature of the ester, this would diminish the product-ee in a one-pot DKR considerably. In contrast, Zr-TUD-1 revealed strong transesterification activity only at high temperature (90 °C). Hardly any benzoin ester was detected at 50 °C or 70 °C. Thus, Zr-TUD-1 makes a selective racemization catalyst up to a temperature of at least 70 °C. Most probably, this can again be explained by the Lewis acidic character of the catalyst, which avoids initiation of transesterification via Brønstedt protonation of the carbonyl group in the acyl donor (which is then attacked by the nucleophilic alcohol).29
DKR of (rac)-benzoin
DKR of (rac)-benzoin was performed in one pot using a combination of Acc-LipTL and metal-TUD-1 as catalysts and vinyl butyrate as acyl donor. In contrast to the reaction set-up of Hoyos et al.,4 use of this most efficient acyl donor was possible, because inactivation of metal-TUD-1 by the emerging volatile acetaldehyde, as observed with Shvo's catalyst, was not to be expected. For optimisation the reaction was investigated using THF, 2-MeTHF and toluene, respectively, as solvents and at temperatures up to 90 °C.Excellent results were obtained with Acc-LipTL and Zr-TUD-1 (Si/Zr = 25) in toluene and equally in 2-MeTHF (Table 3). In toluene 98.4% of the deployed (rac)-benzoin was converted within 5 h yielding a product-ee of 98.5%. Within the same time range, conversion was only slightly lower in 2-MeTHF (96.8%) yielding a still remarkable product-ee of 97%. Interestingly, the situation was controversial when Al-TUD-1 (Si/Al = 4) was used as racemization catalyst, which can most probably be explained by the lower racemization activity of Al-TUD-1 in 2-MeTHF compared to toluene (Table 3) entailing an also lower unspecific transesterification activity.
| Solvent | Rac. catalyst | ee after rac [%]a | Conv. DKR [%]b | ee DKRb [%] |
|---|---|---|---|---|
| a ee of (R)-benzoin, 20 h at 50 °C. b 5 h at 50 °C. c ee of (S)-benzoin butyrate, increased side product formation indicating side activities of the catalyst(s). | ||||
| THF | Al-TUD-1 | 11.9 | 25.1c | 84.2 |
| Zr-TUD-1 | 23.4 | 53.5c | 99.6 | |
| 2-MeTHF | Al-TUD-1 | 7.7 | 85.9 | 96.6 |
| Zr-TUD-1 | 11.4 | 96.8 | 97 | |
| Toluene | Al-TUD-1 | 0.2 | 93.2 | 83.3 |
| Zr-TUD-1 | 1.3 | 98.4 | 98.5 | |
In THF, the DKR was ineffective with both racemization catalysts yielding lower (Al-TUD-1) or only slightly higher (Zr-TUD-1) conversion than theoretically possible in KR (i.e. 50%). This was surprising considering the good results of separate (R)-benzoin racemization in this solvent (see Table 3). The behavior might be connected with side activities of the acidic catalysts under certain conditions (e.g. catalysis of polymerisation)14,30 affecting their racemization activity.
Importantly, it becomes obvious from the performances of the separate and combined catalysts (Tables 2 and 3) that prediction of the best conditions for one pot DKR can hardly be based on the separate reactions. While Al-TUD-1 (Si/Al = 4) in 2-MeTHF leads to faster racemization of benzoin than Zr-TUD-1 (Si/Zr = 25), Zr-TUD-1 is the catalyst of choice for the DKR. Toluene turns out to be the best solvent (with 2-MeTHF as a close second) even though it is only second choice for the KR on its own. This highlights the complexity of combination reactions with two types of catalyst, here heterogeneous and biological, as multi-parameter problems.31
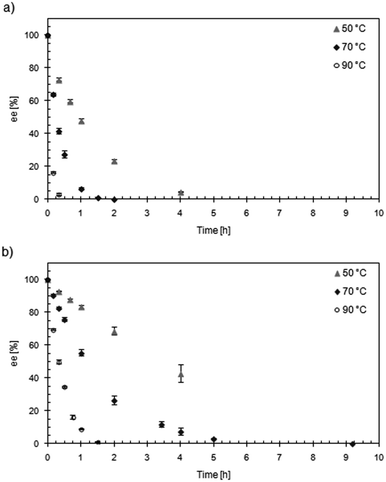
Considering that both KR and racemization, but also unspecific transesterification and biocatalyst inactivation, proceed faster at elevated temperature, DKR of benzoin using Acc-LipTL and Zr-TUD-1 (Si/Zr = 25) in toluene was studied at 50 °C, 70 °C and 90 °C (Fig. 6). At 50 °C, the conversion was over 98% after 5 h with an ee of 98.5%. The reaction time for reaching >98% conversion was reduced to 3.5–4 h when the DKR was performed at 70 °C, while a still very good product-ee of 97.6% was obtained. The minor decrease in ee can certainly be explained by the small unspecific transesterification activity of Zr-TUD-1 at this temperature (see previous section). In agreement with this assumption, the ee dropped to 65.2% at a reaction temperature of 90 °C, where the unspecific transesterification activity of Zr-TUD-1 is considerable. Notably, full conversion was still obtained at this temperature (within 1.5 h) demonstrating a remarkable thermostability of the immobilized biocatalyst under the applied conditions.
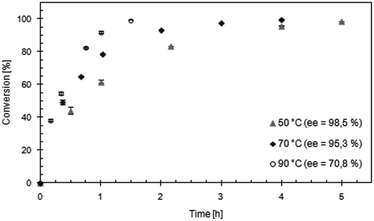 | ||
| Fig. 6 DKR of (rac)-benzoin catalyzed by Acc-LipTL and Zr-TUD-1 (Si/Zr = 25) in toluene at different temperatures. | ||
In view of application in technical synthesis benefitting from repetitive (or continuous) use of the catalysts,31 recyclability of Acc-LipTL and Zr-TUD-1 in DKR was investigated. Repeated batch-cycles were performed in toluene and 2-MeTHF, respectively, at 50 °C. After each cycle the catalysts were separated by filtration and washed with the respective solvent until no residual substrates or product were detectable in HPLC analysis. In comparison of the two solvent systems the reaction time was chosen in a way that both reactions were stopped when full conversion (>99%) was first accomplished in one of the reactions. The maximum reaction time per batch was 24 h.
In 2-MeTHF full conversion of (rac)-benzoin (>99%) was obtained over three batches (Fig. 7) with a runtime of 8 h, 16 h and 24 h, respectively. In the same time intervals DKR in toluene achieved >99% in the first, 96% in the second and only 84% in the third cycle. Product-ee was >97% in both solvents throughout all experiments. The increasing runtimes per cycle required to obtain full conversion in at least one solvent as well as the lower conversion in toluene than in 2-MeTHF within comparable time intervals most probably indicate catalyst deactivation, with a stronger effect exerted by toluene. In principal, this deactivation could concern Acc-LipTL as well as Zr-TUD-1. However, considering that an excellent reusability has been reported for Zr-TUD-1 in the cyclisation of rac-citronellal,15 deactivation of the enzyme catalyst is more likely. This also agrees with the solvent effects on the activity and process stability of Acc-LipTL observed in separate KR (see Table 1).
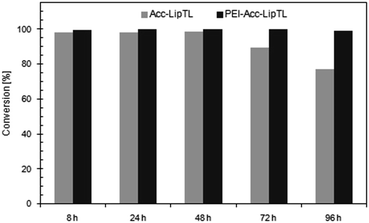
In fact, stability of the reaction system on repetitive use in 2-MeTHF was considerably enhanced (Fig. 8) when the biocatalyst, Acc-LipTL, was stabilized by incubation in a PEI-buffer solution prior to drying as suggested by Guisan et al.32 While in DKR with untreated Acc-LipTL, conversion decreased to 89% in the fourth and 78% in the fifth cycle (each cycle with a runtime of 24 h), use of Acc-LipTL treated with PEI enabled full conversion (>99%) in all cycles. The product-ee remained at >98% and was therefore unaffected by the treatment.
Finally, the actual practicability of the process for synthesis was demonstrated by performing the DKR on a semi-preparative scale using 150 mg racemic benzoin as substrate. The reaction proceeded as smoothly as in small scale (data not shown), reaching 92% conversion within 5 hours and full conversion within less than 20 hours. The product was a colourless oil consisting of enantiopure (ee > 98%) (S)-benzoin butyrate (1H-NMR (400 MHz, CDCl3): 7.92 (m, 2H; Ar-H), 7.55–7.3 (m, 8H; Ar-H), 6.85 (s, 1H; CHO), 2.51–2.39 (m, 2H; CH2), 1.74–1.60 (m, 2H; CH2), 0.98–0.90 (m, 3H; CH3); consult Fig. S1 in the ESI† for the spectrum). The isolated yield after removal of catalysts and solvent was 196 mg corresponding to more than 98%. Thus, the performance of the proposed one-pot system is very promising with regard to synthetic application.
Experimental
Materials
Lipase TL from P. stutzeri was a gift from Meito & Sangyo Co., Ltd. (Tokyo, Japan). Accurel MP1001 was obtained from Membrana (Wuppertal, Germany). TUD-1 catalysts were activated via calcination in the presence of air at up to 600 °C at a temperature ramp of 1 K min−1 and subsequent heating for 10 h at 600 °C. After cooling down to 100 °C the catalysts were stored at 100 °C until use. All chemicals and liquid substrates were purchased from Aldrich (Steinheim, Germany), VWR (Darmstadt, Germany) or Carl-Roth (Karlsruhe, Germany) and used as received. All solvents were dried over activated molecular sieves before use.Reaction analysis
Conversion and enantiomeric excess (ee) were determined via HPLC analysis using a Daicel Chiralpak IA column at a flow of 2.0 mL min−1 of n-hexane/isopropyl alcohol (90/10) at 40 °C. Typical retention times were 2.9 min for (R)-benzoin butyrate, 3.3 min for (S)-benzoin butyrate, 5.8 min for (R)-benzoin and 6.6 min for (S)-benzoin. An exemplary spectrum is given in the ESI (Fig. S1†).Immobilisation of lipase TL
Lipase TL powder was stirred in KPi buffer at room temperature until agglomerates were no more visible. Undissolved material was separated by centrifugation at 5000 rpm for 10 minutes. 500 mg Accurel MP1001 were placed in 3.75 mL ethanol (abs.) for 30 minutes, then 10 mL of lipase solution was directly added. The sealed vessel was placed on an orbital shaker at 340 rpm and 40 °C for 4 h. The immobilisates were afterwards filtered from the solution and washed 2 times with 10 mL immobilisation buffer. After final vacuum filtration the Acc-LipTL particles were dried via storing over silica gel at reduced pressure and room temperature until use.Kinetic resolution (KR)
Typically 20 mg mL−1 (94 mM) (rac)-benzoin was dissolved in dry organic solvent (usually THF). 10 mg of lipase were added to 1 mL of the benzoin solution and the mixture was incubated on a thermoshaker at 1200 rpm and room temperature. After 10 min. KR was started by addition of 6 eq. (related to benzoin) of vinyl butyrate. For sample analysis 10 μL reaction medium were dissolved in 990 μL isopropyl alcohol. If crude lipase powder was used, the diluted samples were centrifugated and the supernatant was analysed. Conversion and enantiomeric excess were determined via HPLC analysis.Racemization of (R)-benzoin
20 mg metal-TUD-1 catalyst were loaded in a dried Schlenk flask under nitrogen atmosphere. (R)-Benzoin was dissolved in toluene at a concentration of 20 mg mL−1; slight heating was required for complete dissolvation. Racemization was started by adding 500 μL of the substrate solution to the metal-TUD-1 catalysts in the Schlenk flask. The reaction vessel was immediately placed in a temperated oil bath (temperature depending on the reaction temperature of following racemization reactions) and mixed with a magnetic stir bar. For sample analysis 10 μL reaction medium were taken under nitrogen counter flow in appropriate time intervals and were dissolved in 990 μL isopropyl alcohol. Enantiomeric excess was determined via HPLC analysis.Dynamic kinetic resolution (DKR)
For characterisation and optimisation, 10 mg Acc-LipTL and 20 mg Metal-TUD-1 (Si/Zr = 25) were loaded in a dried Schlenk flask under nitrogen atmosphere. Benzoin was dissolved in toluene at a concentration of 20 mg mL−1; slight heating was required for complete dissolvation. 500 μL of the substrate solution were added to the lipase and metal-TUD-1 in the Schlenk-flask and the mixture was incubated for 10 minutes. DKR was started by addition of 6 eq. (related to benzoin) of vinyl butyrate and the reaction vessel was immediately placed in a temperature controlled oil bath (temperature depending on the desired reaction temperature) and mixed with a magnetic stir bar. For sample analysis, 10 μL reaction medium were taken under nitrogen counter flow in appropriate time intervals and were dissolved in 990 μL isopropyl alcohol. Conversion and enantiomeric excess were determined via HPLC analysis. For recycling experiments the catalysts (Acc-LipTL and Metal-TUD-1) were separated from the reaction mixture by filtration and washed two times with the solvent providing the reaction medium. After final filtration new substrate solution was added and the reaction was restarted by adding vinyl butyrate under nitrogen atmosphere. The runtime of reaction cycles was adjusted for first reaching a conversion of >98% in one of the solvents. The maximum reaction time was limited to 24 h. For scale-up, the reaction volume was increased to 7.5 mL toluene, using 300 mg Zr-TUD-1 (Si/Zr = 25), 150 mg Acc-LipTL, 150 mg racemic benzoin and 400 mg vinyl butyrate. The reaction was performed under stirring at 50 °C. Samples were taken and defined time intervals and reaction progress was followed via HPLC analysis. After reaching full conversion the catalysts were filtered off and the solvent was removed under reduced pressure.Determination of LipTL stability
A sealed vessel with a volume of 6 mL was continuously operated by using a HPLC pump at a flow rate of 0.5 mL min−1. The benzoin solution (10 mg mL−1 (47 mM) in the given solvent (THF as standard)) with 3 eq. vinyl butyrate was introduced through a pipe and left the reactor by a bleeder pipe. The reaction medium was vigorously mixed with a magnetic stir bar. 100 mg of Acc-LipTL was loaded in the reactor and the reaction was performed until a steady state was reached. For sample analysis 10 μL reaction medium were taken from the outlet stream and dissolved in 990 μL isopropyl alcohol. Conversion and enantiomeric excess were determined via HPLC analysis. From the decrease of the conversion (never exceeded 10%) over time the half life of Acc-Lip TL could be determined.Modification of Acc-LipTL
100 mg Acc-LipTL were incubated in 7.5 mL ethanol (abs.) for 30 minutes. 2 mL of polyethylene imine (PEI)-solution (1% in 200 mmol L−1 KPi) were added to the mixture and the sealed vessel was incubated on an orbital shaker for 1 h at 340 rpm and room temperature. The particles were separated by filtration, washed two times with KPi buffer and dried via storing over silica gel at reduced pressure and room temperature until use.Conclusions
By appropriate preparation and choice of biocatalyst and chemocatalyst, respectively, dynamic kinetic resolution of (rac)-benzoin has been considerably advanced towards preparative scale synthesis. Particularly beneficial were the targeted heterogenization of LipTL through adsorption on Accurel MP1001 and the implementation of heterogeneous Si-based Zr-TUD-1 as racemization catalyst. Both promoted reproducibility of application and recycling of catalysts, and in combination yielded full conversion (>99%) at very high product-ee (>98%) in less than 20 hours. Compatibility in solvent and temperature dependency enabled performance in only one pot. The application range of the reaction system will have to be elucidated in further studies, in view of the documented broad substrate range of LipTL,3–5 however, accessibility of various α-hydroxy ketones via this route can actually be envisaged.Notes and references
- D. Enders and U. Kallfass, Angew. Chem., Int. Ed., 2002, 41, 1743 CrossRef CAS
; Y. Tachibana, N. Kihara and T. Takata, J. Am. Chem. Soc., 2004, 126, 3438 CrossRef PubMed
; S. K. Alamsetti, P. Muthupandi and G. Sekar, Chem.–Eur. J., 2009, 15, 5424 CrossRef PubMed
; P. Muthupandi, S. K. Alamsetti and G. Sekar, Chem. Commun., 2009, 3288 RSC
; P. Hoyos, M. A. Quezada, J. V. Sinisterra and A. R. Alcántara, J. Mol. Catal. B: Enzym., 2011, 72, 20 CrossRef PubMed
; T. Soeta, Y. Tabatake, K. Inomata and Y. Ukaji, Tetrahedron, 2012, 68, 894 CrossRef PubMed
.
- P. Hoyos, J. V. Sinisterra, F. Molinari, A. R. Alcantara and P. Dominguez-de-Maria, Acc. Chem. Res., 2010, 43, 288 CrossRef CAS PubMed
.
- P. Hoyos, V. Pace, J. V. Sinisterra and A. R. Alcántara, Tetrahedron, 2011, 67, 7321 CrossRef CAS PubMed
.
- P. Hoyos, M. Hernandez, J. V. Sinisterra and A. R. Alcántara, J. Org. Chem., 2006, 71, 7632 CrossRef CAS PubMed
.
- P. Hoyos, A. Buthe, M. B. Ansorge-Schumacher, J. V. Sinisterra and A. R. Alcántara, J. Mol. Catal. B: Enzym., 2008, 52–53, 133 CrossRef CAS PubMed
.
- O. Pamies and J. E. Bäckvall, Curr. Opin. Biotechnol., 2003, 52–53, 133 Search PubMed
.
- S. Agrawal, E. Martínez-Castro, R. Marcos and B. Martín-Matute, Org. Lett., 2014, 16, 2256 CrossRef CAS PubMed
.
- M. Persson, I. Mladenoska, E. Wehtje and P. Adlercreutz, Enzyme Microb. Technol., 2002, 31, 833 CrossRef CAS
; A. Salis, E. Sanjust, V. Solinas and M. Monduzzi, J. Mol. Catal. B: Enzym., 2003, 24–25, 75 CrossRef
; P. Torres, D. Reyes-Duarte, N. Lopez-Cortes, M. Ferrer, A. Ballesteros and F. J. Plou, Process Biochem., 2008, 43, 145 CrossRef PubMed
.
- B. Al-Duri and Y. P. Yong, Biochem. Eng. J., 2000, 4, 207 CrossRef CAS
; L. L. Cao, Carrier-bound immobilized enzymes, Wiley-VCH, Weinheim, 2005 RSC
; U. Hanefeld, L. Gardossi and E. Magner, Chem. Soc. Rev., 2009, 38, 453 RSC
.
- Y. Aoyagi, N. Agata, N. Shibata, M. Horiguchia and R. M. Williams, Tetrahedron Lett., 2000, 41, 10159 CrossRef CAS
; Y. Aoyagi, A. Iijima and R. M. Williams, J. Org. Chem., 2001, 66, 8010 CrossRef PubMed
.
- J. M. Palomo, G. Munoz, G. Fernandez-Lorente, C. Mateo, R. Fernandez-Lafuente and J. M. Guisan, J. Mol. Catal. B: Enzym., 2002, 19, 279 CrossRef
.
- A. Maraite, P. Hoyos, J. D. Carbeillera, A. C. Cabrera, M. B. Ansorge-Schumacher and A. R. Alcántara, J. Mol. Catal. B: Enzym., 2013, 87, 88 CrossRef CAS PubMed
.
- M. M. Diogo, S. Silva, J. M. S. Cabral and J. A. Queiroz, J. Chromatogr. A, 1999, 849, 413 CrossRef CAS
; A. C. Dias-Cabral, A. S. Ferreira, J. Phillips, J. A. Queiroz and N. G. Pinto, Biomed. Chromatogr., 2005, 19, 606 CrossRef PubMed
.
- V. Pace, P. Hoyos, L. Castoldi, P. Dominguez-de-María and A. R. Alcántara, ChemSusChem, 2012, 5, 1369 CrossRef CAS PubMed
.
- A. Ramanathan, M. C. C. Villalobos, C. Kwakernaak, S. Telalovic and U. Hanefeld, Chem.–Eur. J., 2008, 14, 961 CrossRef CAS PubMed
.
- S. Wuyts, D. E. D. Vos, F. Verpoort, D. Depla, R. D. Gryse and P. A. Jacobs, J. Catal., 2003, 219, 417 CrossRef CAS
; W. H. Kim, R. Karvembu and J. Park, Bull. Korean Chem. Soc., 2004, 25, 931 CrossRef
; A. Zsigmond, A. Kecskemeti, K. Bogar, F. Notheisz and E. Mernyak, Catal. Commun., 2005, 6, 520 CrossRef PubMed
; A. Berkessel, M. L. Sebastian-Ibarz and T. N. Muller, Angew. Chem., Int. Ed., 2006, 45, 6567 CrossRef PubMed
; S. Wuyts, J. Wahlen, P. A. Jacobs and D. E. D. Vos, Green Chem., 2007, 9, 1104 RSC
; L. F. A. Costa, F. Lemos, F. R. Ribeiro and J. M. S. Cabral, Catal. Today, 2008, 133, 625 CrossRef PubMed
; A. Parvulescu, J. Janssens, J. Vanderleyden and D. E. D. Vos, Top. Catal., 2010, 53, 931 CrossRef
; Y. M. Cheng, G. Xu, J. P. Wu, C. S. Zhang and L. R. Yang, Tetrahedron Lett., 2010, 51, 2366 CrossRef PubMed
; Y. M. Cheng, G. Xu, J. P. Wu and L. R. Yang, Chin. J. Org. Chem., 2010, 30, 1695 Search PubMed
.
- Y. Z. Zhu, K. L. Fow, G. K. Chuah and S. Jaenicke, Chem.–Eur. J., 2007, 13, 541 CrossRef CAS PubMed
.
- P. Odman, L. A. Wessjohann and U. T. Bornscheuer, J. Org. Chem., 2005, 70, 9551 CrossRef PubMed
.
- A. Ramanathan, D. Klomp, J. A. Peters and U. Hanefeld, J. Mol. Catal. A: Chem., 2006, 260, 62 CrossRef CAS PubMed
.
- Y. Wan and D. Y. Zhao, Chem. Rev., 2007, 107, 2821 CrossRef CAS PubMed
.
- S. Telalovic, A. Ramanathan, G. Mul and U. Hanefeld, J. Mater. Chem., 2010, 20, 642 RSC
.
- S. Telalovic, J. F. Ng, R. Maheswari, A. Ramanathan, G. K. Chuah and U. Hanefeld, Chem. Commun., 2008, 4631 RSC
.
- S. Telalovic, A. Ramanathan, J. F. Ng, R. Maheswari, C. Kwakernaak, F. Soulimani, H. C. Brouwer, G. K. Chuah, B. M. Weckhuysen and U. Hanefeld, Chem.–Eur. J., 2011, 17, 2077 CrossRef CAS PubMed
.
- C. Simons, U. Hanefeld, I. W. C. E. Arends, R. A. Sheldon and T. Maschmeyer, Chem.–Eur. J., 2004, 10, 5829 CrossRef CAS PubMed
; S. Telalovic, S. K. Karmee, A. Ramanathan and U. Hanefeld, J. Mol. Catal. A: Chem., 2013, 368, 88 CrossRef PubMed
.
- A. Ramanathan, R. Maheswari and U. Hanefeld, J. Catal., 2006, 242, 82 CrossRef PubMed
.
- M. P. Pachamuthu, V. V. Srinivasan, R. Maheswari, K. Shanthi and A. Ramanathan, Appl. Catal., A, 2013, 462, 143 CrossRef PubMed
.
- J. ten-Dam, D. Badloe, A. Ramanathan, K. Djanashvili, F. Kapteijn and U. Hanefeld, Appl. Catal., A, 2013, 468, 150 CrossRef CAS PubMed
.
- S. Wuyts, K. de-Temmermann, D. E. D. Vos and P. A. Jacobs, Chem.–Eur. J., 2004, 11, 386 CrossRef CAS PubMed
.
- U. Schuchardt, R. Sercheli and R. M. Vargas, J. Braz. Chem. Soc., 1998, 9, 199 CrossRef CAS PubMed
.
- D. Klomp, T. Maschmeyer, U. Hanefeld and J. A. Peters, Chem.–Eur. J., 2004, 10, 2088 CrossRef CAS PubMed
.
- L. Veum and U. Hanefeld, Chem. Commun., 2006, 825 RSC
.
- J. M. Guisan, P. Sabuquillo, R. Fernandez-Lafuente, G. Fernandez-Lorente, C. Mateo, P. J. Halling, D. Kennedy, E. Miyata and D. Re, J. Mol. Catal. B: Enzym., 2001, 11, 817 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra06751g |
| This journal is © The Royal Society of Chemistry 2014 |