Photocatalytic dye degradation properties of wafer level GaN nanowires by catalytic and self-catalytic approach using chemical vapor deposition†
Abstract
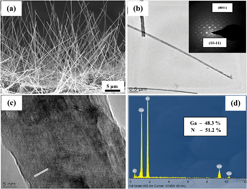
We report the photocatalytic dye degradation properties of self-assembled gallium nitride (GaN) nanowires grown using chemical vapor deposition. GaN nanowires have been fabricated using Ni and Au as the catalyst in addition to the self-catalytic approach. The defect engineered multiband GaN nanowires were subjected to the photocatalytic decay of methylene blue (MB), a common dye in the dying industry, under UV and visible light. Ni-catalyst assisted GaN nanowires possess the maximum degradation of 93% under UV irradiation as correlated with the strong UV band edge emission at 3.42 eV. Interestingly, self-catalytic GaN nanowires show higher photocatalytic activity of 89% under visible irradiation than the catalyst assisted nanowires because of the presence of radiative surface defects in visible region as evidenced by photoluminescence studies. The nanowires exhibit enhanced photocatalytic decay as compared to epitaxial layer due to high surface area and active sites of one-dimensional nanostructures. Detailed analysis further demonstrates the stable photocatalytic activity of the GaN NWs for several cycles against photocorrosion. This work establishes the use of wafer level GaN NWs as a viable photocatalyst for visible light driven decay of organic pollutants.

 Please wait while we load your content...
Please wait while we load your content...