Recent advances in the synthesis of quinolines: a review
Shraddha M. Prajapati
,
Kinjal D. Patel
,
Rajesh H. Vekariya
,
Shyamali N. Panchal
and
Hitesh D. Patel
*
Department of Chemistry, School of Sciences, Gujarat University, Ahmedabad, Gujarat, India. E-mail: drhiteshpatel1@gmail.com; Fax: +91-079-26308545; Tel: +91-079-26300969
First published on 17th April 2014
Abstract
Quinolines have become important compounds because of their variety of applications in medicinal, synthetic organic chemistry as well as in the field of industrial chemistry. In recent years there are greater societal expectations that chemists should produce greener and more sustainable chemical processes. This review article gives information about the green and clean syntheses using alternative reaction methods for the synthesis of quinoline derivatives. The article includes synthesis by microwave, using clay or some other catalyst which could be recycled and reused, one-pot reaction, solvent-free reaction conditions, using ionic liquids, ultrasound promoted synthesis and photocatalytic synthesis (UV radiation).
Introduction
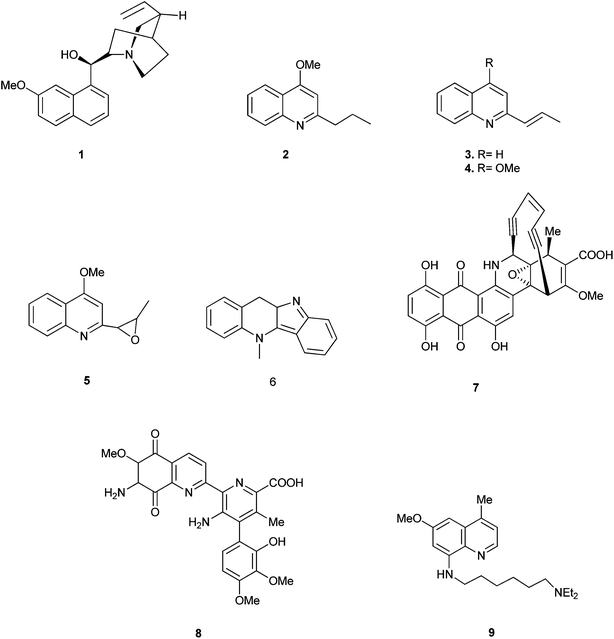
Quinoline is one of the most important N-based heterocyclic aromatic compounds. Quinolines recently have been caught the attention of researchers because of their broad range of activities and of course for their wide applications too.The main sources of quinoline include petroleum, coal processing, wood preservation and shale oil. The quinoline derivatives occur in various natural products, especially in alkaloids. In 1820, quinine (1) was isolated from the bark of the cinchona tree which replaced the use of crude bark for the treatment of malaria. Other quinoline derivatives (2–9) having various activities were also isolated from different plant species.1 (Fig. 1).
Quinoline was first extracted from coal tar in 1834 by Friedlieb Ferdinand Runge. Coal tar remains the principal source of commercial quinoline.2
Activities and applications
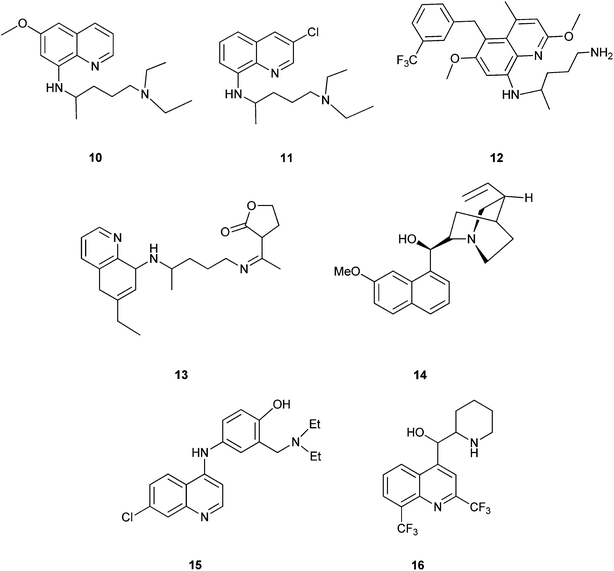
Quinoline derivatives in general are known to have a broad range of applications in medicinal, bioorganic, and industrial chemistry as well as in the field of synthetic organic chemistry.Their derivatives have been found to possess various biological activities like anti-malarial, anti-bacterial, anti-fungal, anti-asthmatic, antihypertensive, anti-inflammatory, and anti-platelet activity.3 They also exhibit anti-tubercular4 and immune depressing4 activities. There are a few promising compounds with the quinoline ring system, like pamaquine (10), chloroquine (11), tafenoquine (12), bulaquine (13), quinine (14) and mefloquine (16) as an antimalarial agents, and amodiaquine (15) as an antimalarial and anti-inflammatory agent (Fig. 2).5–7
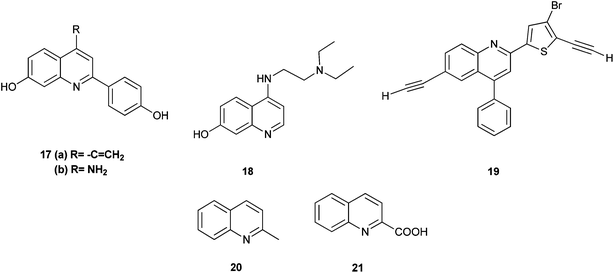
The 2-arylquinoline derivatives (17a) and (17b) show selectivity in binding to the estrogen receptor β (ER β), which plays an important role in the development, maintenance, and function of the mammalian reproductive system, as well as in non-sexual tissues.8
4-[2-(Diethylamino) ethylamino] quinolin-7-ol (18) containing nitrogen at position 4 exhibits antiplasmodial activity.9 Polysubstituted quinoline derivatives such as 8-hydroxyquinoline and quinoline-8-thiol have been used to produce metal complexes which emit light.10 2-(4-Bromo-5-ethylnylthiophen-2-yl)-6-ethynyl-4-phenylquinoline (19) has been applied in sensors and light emitting diodes.11
Some quinoline derivatives such as quinalidine (20) and quinaldic acid (21) show activity as corrosion inhibitors for mild steel in hydrochloric acid.4 (Fig. 3).
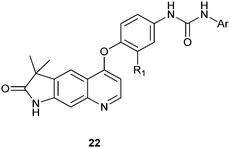
In recent years some quinoline derivatives (22) shown below, which have explored the structural modification of Sorafenib, where synthesized as novel Raf kinase inhibitors with more potent and selective antitumor activities.12
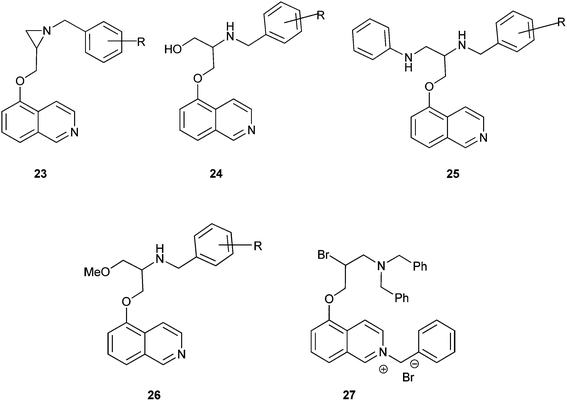
Aziridine–isoquinoline hybrids and their ring-opening products shown in Fig. 4exhibit potent antiplasmodial activity.13
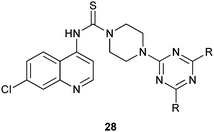
A new series of hybrid conjugates of N-(7-chloroquinolin-4-yl) piperazine-1-carbothioamide and 1,3,5-triazine derivatives have considerable antimalarial activity against both wild and mutant parasites with marked variations on changing the pattern of substitution. Such derivatives also show excellent antibacterial activity against several Gram-positive and Gram-negative microorganisms.14,15
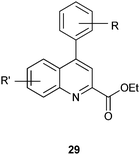
4-Arylquinoline-2-carboxylate derivatives show antiprotozoal activity against the pathogenic parasite Toxoplasma gondii.16
Many quinoline derivatives are found to have applications as agrochemicals17 as well as use in the study of bio-organic and bio-organometallic3 processes. They are also used in manufacturing dyes, food colorants, pH indicators and other organic compounds. In additional to this they have also been used as ligands for the preparation of OLED phosphorescent complexes18 and with conjugated polymers used as a selective chemo-sensors of the fluoride and metal ions.19,20
Due to such a wide range of applicability, there has been increasing interest in the development of efficient methodologies for the synthesis of quinoline derivatives.
Conventional methods of synthesis
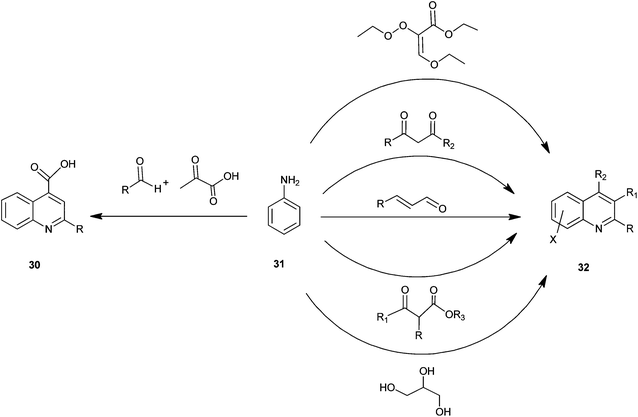
A number of preparations have been known since the late 1800s for the synthesis of quinoline and its derivatives (Fig. 5).The structural core of quinoline has been generally synthesized by various conventional named reactions such as Skraup, Doebner-von Miller, Friedlander, Pfitzinger, Conrad-Limpach, Combes synthesis.1
Though many of these methods are very effective, they often involve the use of various acids or reagents that are not environmentally compatible, produce a large amount of waste and require longer reaction times.17 Moreover, many of these methods give relatively large amounts of undesirable by-products whose removal is tedious and often wasteful and are also not satisfactory with respect to operational simplicity to isolate the yield. Thus, it has become very important to follow methods which could be considered as a better and eco-friendly viable ‘green synthetic methods’.
This green chemistry approach provides a way to design products in a simplified manner and has less feedstock, minimum waste, low energy consumption, less hazardous, renewable materials, high atom economy, reduce reaction steps and green catalysts that improve the efficiency of the reaction.
Clean chemical synthesis using alternative reaction methods includes:
(1) Alternative reaction media for the synthesis using supercritical fluids, ionic liquids, water, polyethylene glycol and solvent-free or grinding methods.
(2) Alternative energy sources like microwaves (fast and homogeneous heating by microwave irradiation), ultrasound and sunlight/UV.
(3) Catalysts which may be clay or other green catalysts which could be recycled and reused.
Various reaction schemes
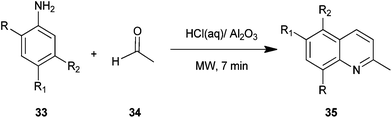
Safari et al. described a procedure for preparation of quinaldine derivatives (35) from aniline derivatives (33) and acetaldehyde (34) under microwave irradiation without any solvent (Scheme 1).22 In this method they tried different Bronsted acids but found hydrochloric acid appeared to be the best catalyst for this reaction, showing the highest yield. Moreover the yield of the product was not affected by the nature of substituent in this reaction.The method presents a simple and useful synthetic process for quinaldines because of high yields, short reaction time, a straight forward, easy work-up procedure, the use of microwave irradiation as the novel efficient source of energy and the use of molecular oxygen as a green oxidant.
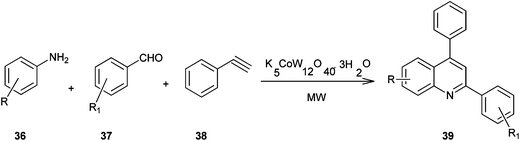
A method for the synthesis of quinoline derivatives and bis-quinoline derivatives (39) including a microwave-assisted, one-pot-three-component reaction between aromatic amines, (36) aromatic aldehydes (37) and phenylacetylene (38) in the presence of catalytic amounts of potassium dodecatungstocobaltate trihydrate (K5CoW12O40·3H2O) has been demonstrated by Anvar and his co-workers (Scheme 2).23 They were first to report the use of polyoxometalates (POMs) as catalysts for the one-pot three-component synthesis of quinolines and bis-quinolines under microwave irradiation.
The catalyst could be easily recovered by simple filtration and could be reused for several cycles without any significant loss of its catalytic activity. Moreover no metal was detected in the final product which confirms the green nature of the present method. This makes the method useful and attractive for the synthesis of quinoline derivatives.
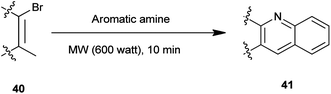
A synthesis of steroidal and nonsteroidal quinoline derivatives (41) has been established by Gogoi et al. (Scheme 3).17 In this method steroidal quinoline derivatives were synthesized from a one-pot reaction of steroidal β-bromovinyl aldehydes (40) and arylamines in high yield using microwave irradiation without the use of a catalyst and in a solvent-free condition. This methodology offers an environment friendly ‘green’ alternative organic synthesis.
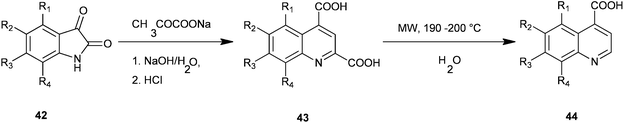
Facile microwave-assisted processes suitable for the preparation of a series of quinoline-4-carboxylic acids (44) have been introduced by Zhu and co-workers (Scheme 4).24 In this Pfitzinger type of reaction a condensation reaction between isatins (42) and sodium pyruvate to give quinoline-2,4-dicarboxylic acid (QDC) (43) is carried out under microwave conditions which optimise reaction solvent, time and temperature. The subsequent decarboxylation reaction of QDCs in water instead of toxic nitrobenzene under MW was also promoted successfully.
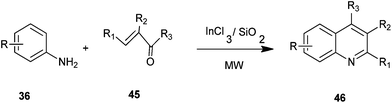
Ranu et al. developed a simple and efficient procedure for the synthesis of 4-alkylquinoline derivatives (46) by a one-pot reaction of anilines (36) with alkyl vinyl ketones (45) on the surface of a silica gel inseminated with indium(III) chloride under microwave irradiation without any solvent (Scheme 5).25 The main advantages of this procedure are: operational simplicity, fast reaction, high yield and general applicability. These advantages accommodate a variety of substitution patterns.
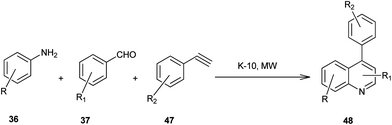
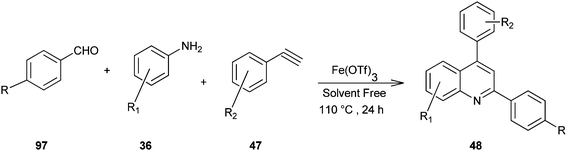
Microwave-assisted solid acid-catalyzed syntheses of substituted quinoline derivatives (48) have been discussed by Kulkarni and his colleagues (Scheme 6).26 The quinoline derivatives were synthesized by a multicomponent reaction of anilines (36), aldehydes (37) and terminal aryl alkynes (49). The reaction was catalyzed by montmorillonite K-10, a strong and environmentally benign solid acid. The multicomponent approach yields products with nearly 90% atom economy in excellent yields in a matter of minutes. The use of microwave activation reduces the reaction time significantly.
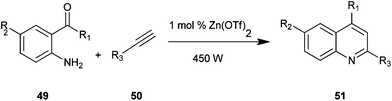
Quinoline derivatives (51) were synthesized by employing amino acetophenone (41) and phenylacetylene (50) in the presence of Zn(OTf)2 as an effective catalyst under microwave irradiation, as presented by Praveen and co-workers (Scheme 7).27
The advantage of this method is that the reaction is amenable to alkynes containing alkyl, aromatic and heteroaromatic groups. Good yields, a short reaction time, operational simplicity, low catalyst loading and wide substrate scope are the significant advantages of this reaction from synthetic viewpoint.
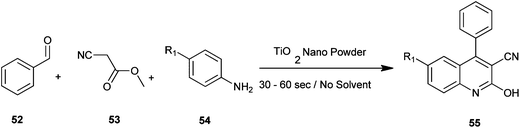
Naik et al. have reported a rapid and efficient method for the synthesis of various carbonitrile quinoline/benzo[h]quinoline derivatives (55) by utilizing benzaldehyde (52), methyl cyanoacetate (53) and aromatic amine (54) with nanostructured TiO2 photocatalysts under solvent-free conditions under microwave irradiation (Scheme 8).28
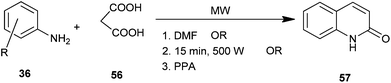
3-Unsubstituted 4-hydroxyquinolin-2(1H)-one (57) was synthesized using substituted aromatic amine (36) and malonic acid (56) under microwave irradiation in dimethylformamide,29 without employing any solvent30 and using polyphosphoric acid (PPA)31 (Scheme 9). Operational simplicity and high yield in significantly very short reaction time make this procedure a useful and attractive alternative to the currently available methods.
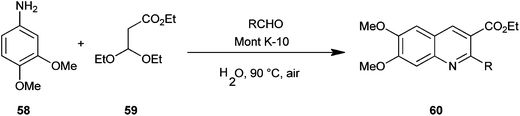
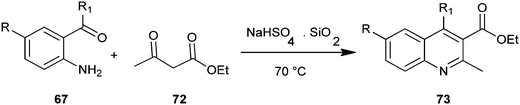
Reddy et al. introduced a three-component one-pot reaction between 3,4-dimethoxyaniline (58), aldehydes and ethyl-3,3-diethoxypropionate (59) to a quinoline derivative (60) by using montmorillonite K-10 (Mont K-10) as a green catalyst by utilizing the oxygen of air and water (Scheme 10).21 Montmorillonite K-10 (Mont K-10) was found to be more effective compared to other Lewis acids as the expected product was isolated in good yield. Moreover montmorillonite K-10 was recovered by simple filtration and reused easily, while the use of water as a solvent makes this reaction eco-friendly.
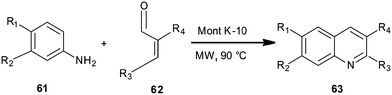
Montmorillonite K10 clay-catalyzed synthesis of quinoline derivative (63) has been disclosed by Nagendrappa et al. by employing aniline derivatives (61) and cinnamaldehyde (62) (Scheme 11).32 The mechanism follows a domino process involving cyclization followed by dehydration and then after oxidation delivers quinolines. The reaction was carried out under solvent-free conditions and with the assistance of microwave irradiation.
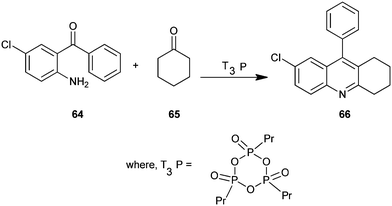
A new, convenient, efficient and eco-friendly protocol for the synthesis of a polysubstituted quinoline derivative (66) under mild condition has been described by Jida et al. (Scheme 12).33 A wide variety of new products were readily prepared in the presence of propylphosphonic anhydride (T3P) in short reaction times and in excellent yields. Here T3P is used as a mild water scavenger catalyst in this coupling reaction. In addition, this non-toxic T3P offers several advantages over traditional reagents, such as low toxicity, commercial scale availability, low price, low epimerization tendency, high selectivity yields, excellent purity, a broad functional group, and easy work-up procedures. The simplicity and cost-effectiveness of this methodology are attractive for large scale synthesis.
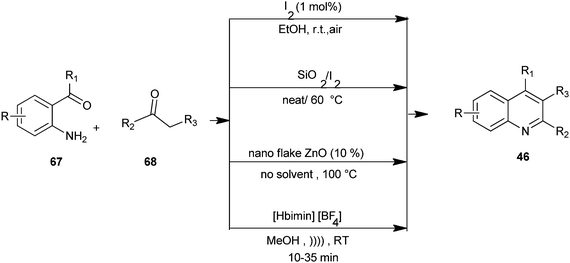
Mild and efficient routes for the synthesis of quinoline derivatives and polycyclic quinoline derivatives (46) by utilizing substituted o-amino acetophenone derivative (67) and enolisable ketone (68) with molecular iodine as a catalyst in ethanol,34 combining iodine and silica gel under solvent-free conditions,35 a Friedlander heteroannulation method by using nano ZnO as a mild, non-volatile, non-corrosive and efficient catalyst which provides regiospecific synthesis under solvent-free conditions,36 and using ionic liquid [Hbim][BF4] under ultrasound at room temperature.37 These methods avoid the use of hazardous acids or bases and harsh reaction conditions. The advantages of these methods include good substrate generality, the use of inexpensive reagents and catalysts under mild conditions, and experimental operational ease (Scheme 13).
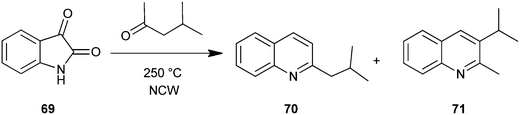
Gibson et al. used near critical water (NCW) as a medium for the organic synthesis of nitrogen heterocyclic compounds. The potential for solvent recycling was also demonstrated. In this method carbonyl compound with isatin (69) in NCW form the substituted quinoline derivative (70–71) via in situ decarboxylation (Scheme 14).38 Hot pressurizing NCW to make it more ionized makes it a very good dehydrating media suited for isatin opening, condensation and cyclodehydration with carbonyls.
A highly efficient, cost-effective and environmentally benign protocol for the synthesis of 2,4,6-trisubstituted quinoline derivative (73) by using NaHSO4·SiO2 as a heterogeneous and reusable catalyst has been disclosed by Vu and co-workers (Scheme 15).8 This procedure is operationally simple and can be an alternative to the existing protocols for the synthesis of tri-substituted quinoline derivatives. From a practical point of view, the catalyst is easier to prepare from readily available reagents and like other heterogeneous catalysts, it can also be reused.
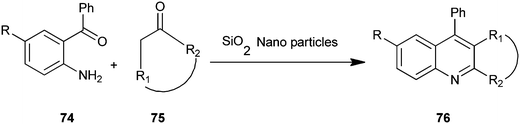
A microwave-assisted procedure for quinoline derivative (76) has been reported by Shekouhy and co-workers (Scheme 16).39 The reaction between 2-aminoaryl ketones (74) and carbonyl compounds (75) in the presence of silica nano-particles (NPs) as catalysts under microwave irradiation give high yields of quinoline derivatives. Silica nano-particles gave best results compare to CaO, MgO, Al2O3 and SiO2. Silica NPs, a highly microporous solid, offer a wide range of active sites and often can be regenerated if deactivated during the reaction. On the other hand, reducing catalytic substances to nanometers in size greatly increases the surface area available per gram and ultimately the catalytic activity. The NPs catalyst can be reused without loss of activity even after recycling fourteen times.
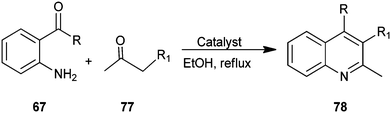
A one-pot, mild, efficient, and environmentally benign protocol has been developed by Chermahini et al. for the synthesis of a quinoline derivative (78) catalyzed by Montmorrilonite K-10, zeolite, nano-crystalline sulfated zirconia (nano-crystalline SZ) in high yields (Scheme 17).40 The mild reaction conditions, easy work-up, clean reaction profiles, lower catalyst loading and cost efficiency make this approach an interesting alternative to the existing methods.
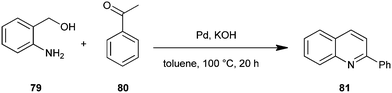
Ren et al. have disclosed a method in which 2-aminobenzyl alcohol (79) reacts with ketones (80) in toluene or polyethylene glycol (PEG-2000) by employing a palladium catalyst along with KOH to isolate the corresponding quinoline derivative (81) in good yields (Scheme 18).41 The catalytic system could be recovered and reused five times without any loss of catalytic activity.
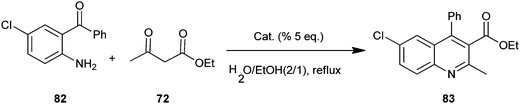
The use of some new metal dodecyl sulfates as a catalyst in the synthesis of quinoline derivatives (83) by a Friedlander protocol in aqueous media have been developed by Salehi et al. (Scheme 19).42 In this synthesis metal dodecyl sulfates were used in reaction between o-aminoaryl ketones (82) and ketones or β-diketones (72). The authors prepared some combined Lewis acid-surfactant catalysts (LASC) and tried to use them for the synthesis. They found zirconium tetrakisdodecyl sulfate Zr(DS)4 superior to all other prepared metal dodecyl sulfates. Zr(DS)4 was recovered easily through separation by a centrifuge and reused in aqueous media.
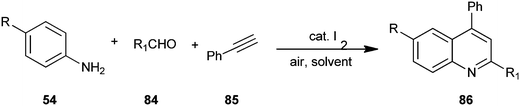
An air-mediated metal-free one-pot and three-component green synthetic method for obtaining a quinoline derivative (86) by utilizing 4-substituted aniline (54), aldehyde derivative (84), ethynylbenzene (85) with molecular iodine as a catalyst has been reported by Li et al. (Scheme 20).43 Here molecular iodine acts as a mild Lewis acid.
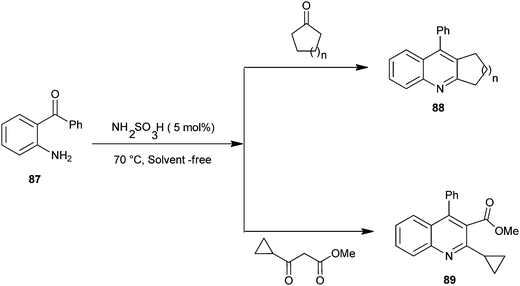
The synthesis of quinoline derivatives and polycyclic quinoline derivatives (88–89) via a Friedlander condensation between 2-aminoarylketones (87) with enolisable ketones by using the recyclable heterogeneous catalyst sulfamic acid have been described by Rao and colleagues (Scheme 21).44 The ease of recovery and reuse of this novel catalyst makes this method quite simple, convenient and environmentally benign for the synthesis of highly functionalized quinoline derivatives.
The one-pot synthesis of 2- or 3-mono-substituted, 2,3-disubstituted quinoline derivatives and also other bi- or tricyclic quinoline derivatives has been presented by Li et al. (Scheme 22).45 The reaction was applicable to a wide variety of ketones and aldehydes. The biggest advantage of this method is use of inexpensive, readily available reagents and solvents that do not require oxygen or moisture-free conditions.
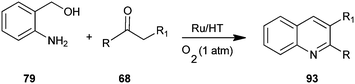
Motokura et al. have presented a one-pot process for the synthesis of quinoline derivative (93) by using (2-aminophenyl)methanol (79) and enolisable ketone (68) with Ru-grafted hydrotalcite (Ru/HT), a multifunctional heterogeneous catalyst (Scheme 23).46 The presented catalytic system improves on previously reported catalytic systems through advantages like high catalytic activity, wide applicability to various carbonyl compounds, no need for homogeneous bases and the use of molecular oxygen as a green oxidant. This is the first reported one-pot quinoline synthesis using heterogeneous catalysts.
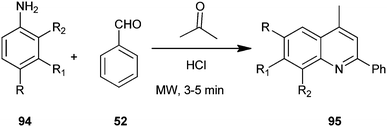
A one-pot, solvent free, microwave-assisted, multi-component reaction for the synthesis of a quinoline derivative (95) without any solvent by utilizing substituted aniline (94), acetone and benzaldehyde (52) on the surface of alumina impregnated with hydrochloric acid has been developed by Mirza et al. (Scheme 24).47 Hydrochloric acid appeared to be the best catalyst for this reaction, showing the highest yield and was preferred compared to sulfuric acid because of its higher safety, environmental friendliness and lower price. The advantages like high yield, short reaction time, straight forward and easy work-up procedure makes the method completely eco-friendly, fast, simple, and highly efficient.
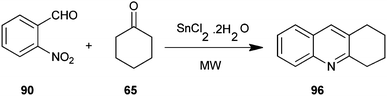
An efficient microwave assisted, one-pot, solvent-free synthesis of substituted quinoline derivative (96) from o-nitrobenzaldehyde (90) and enolizable ketones (65) using SnCl2·2H2O as the reductant has been introduced by Chaudhuri and co-workers (Scheme 25).48 This method is relatively faster and it affords the desired products in respectable yields.
Yao et al. have described a novel application of highly stable Fe(OTf)3 as an efficient catalyst for carbon–carbon bond formation via the activation of a terminal alkyne C–H bond under solvent-free conditions for the synthesis of a quinoline derivative (48) (Scheme 26).49 Furthermore, the catalyst was easily recovered from the reaction mixture and reused many times with only a little loss of activity. Other catalysts such as FeCl3, Fe(OTf)2, FeSO4, Fe(NO3)3 and Fe2(SO4)3 were examined in this reaction, but they did not behave as efficiently as Fe(OTf)3 in terms of yield of the products. It was also noted that the reaction in solvent-free conditions was more favorable compared to reactions with various organic solvents.
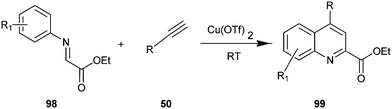
The synthesis of quinoline-2-carboxylate derivative (98) by Cu(OTf)2 catalyst via the intermolecular addition of alkyne (50) onto imines (99) and subsequent intermolecular ring closure by arylation have been described by Huang et al. (Scheme 27).50 In addition, various catalyst such as Cu(OAc)2, Cu(acac)2, Cu(tmhd)2, CuI, Cu(OTf)2 were examined by authors in this reaction with different organic solvents like DCM, 1,4-dioxane, toluene, furan, DMSO and DMF. However the best result was obtained when the reaction was carried out using Cu(OTf)2 in DCM. The efficiency of this system allowed the reactions to be carried out at room temperature.
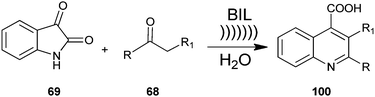
A method for producing a quinoline derivative (100) using ultrasound and an ionic liquid has been established by Kowsari et al. a two-component, one-pot, condensation reaction of isatin (69) with enolisable ketone (68) yields the quinoline derivative (100) (Scheme 28).51 Authors examined the various solvents’ effect on the reaction. The results showed that the reaction is favored in water. Also, the ionic liquid could be recovered and reused many times without loss of its activity. In addition, a simple process, high selectivity, short reaction time, use of cheap and environmentally benign solvent and the reusability of the aqueous media are the superior advantages of this protocol.
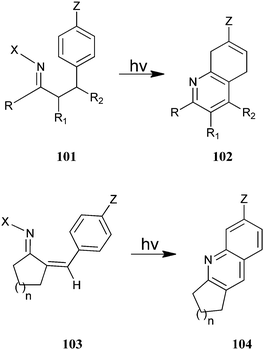
Austin et al. have described the photosynthesis of a substituted annulated quinoline derivative (102–104) from (101) and (103) (Scheme 29).52 This photocyclization–elimination process provides a convenient route for the synthesis of a variety of substituted 2,3-dihydro-1H-cyclopenta[b] quinoline derivatives from readily accessible precursors. Reactions of meta-substituted precursors were highly regioselective, with alkyl substituents, which afforded 5-substituted 2,3-dihydro-1H-cyclopenta[b]quinolines. In addition, substrates containing powerful electron-donating substituents generally afford 7-substituted products in high yields.
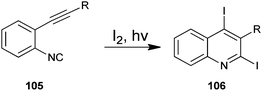
The photochemical cyclization method for the synthesis of 2,4-diiodoquinoline derivative (105) by the cyclization of o-alkynylaryl isocyanide (106) with iodine has been developed by Mitamura and co-workers (Scheme 30).53 Which are difficult to synthesize by using other existing methods. For a comparative study authors employed various organic solvents such as chloroform, methanol, acetone, acetonitrile, ethyl acetate, tetrahydrofuran, toluene, n-hexane and triethyl amine. However the best results were obtained in terms of the yield of the products when chloroform was used as a solvent. This photochemical reaction was also carried out with various cyclizing agents such as Br2, N-chlorosuccinimide (NCS), N-bromosuccinimide (NBS) and N-iodosuccinimide (NIS) but they were not found to be efficient catalysts for this protocol. This eco-friendly green reaction proceeds under mild conditions and readily affords the appropriate products in high to excellent yields.
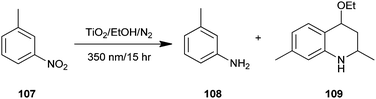
A one-pot synthesis of 4-ethoxy-l,2,3,4-tetrahydroquinoline (109) from a heterogeneous solution of nitroarene (107), ethanol and TiO2 upon irradiation of UV light has been reported by Joo and colleagues (Scheme 31).54 In addition, substrates have either oxygen or amino substituents like m-nitroanisole etc., in such cases the reaction proceeds rather slowly compared to those having alkyl substituents under the same reaction conditions. An added advantage of this protocol is that quinoline derivatives were directly synthesized from the nitro nitroarenes instead of aminoarenes using environmentally friendly conditions.
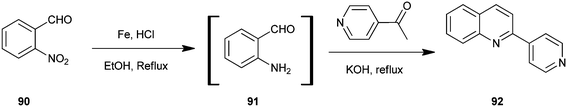
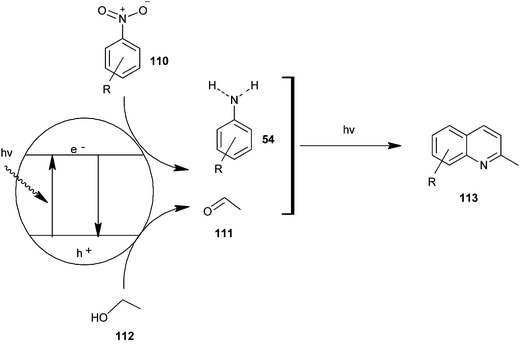
Selvam et al. have demonstrated the photo catalytic conversion of nitrobenzene (110) to 2-methylquinoline derivative (113) in absolute ethanol using TiO2 as a photo catalyst. In this reaction exited electrons are trapped by the metal which is used to dope TiO2; this enhances the charge separation between hole and electron. Holes cause the oxidation of alcohol (112) to aldehyde (111) and the trapped electrons are consumed for the reduction of nitrobenzene to aniline by H+ formed during the oxidation of alcohol. The further reaction of aniline and aldehyde gives quinaldine. This photo reaction is only possible in the presence of TiO2. In 2009 Selvam et al. prepared substituted quinoline derivatives and other heterocycles by TiO2 nanoparticles via this photocatalytic process.55 In 2010 they synthesized quinaldines Au-loaded TiO2.56 In 2011 they synthesized nanosized silver particles loaded TiO2 to get materials with enhanced adsorption and photocatalytic performance.57 In 2012 N-doped TiO2 using a new nitrogen precursor hydrazine hydrate has been synthesized by a simple wet method by Selvam et al. (Scheme 32). They concluded that N–TiO2 is more efficient than other metal-doped catalysts in quinaldine synthesis under visible light. Therefore, this process has the potential to enable a more sustainable quinaldine synthesis from nitrobenzene in UV and visible light.55
Conclusion
Since quinoline and its derivatives possess a wide spectrum of pharmacological activities and are also utilized as ligands in various biologically-modelled transition metal complexes, a number of methods have been developed from time to time for their synthesis by microwave-assisted, ultrasound-promoted, or heterogeneous acid-catalyzed methods, in UV light or solvent-free conditions and many more. We have made here efforts to compile most of these methods that have been reported in the literature. This review will be very useful to the researcher working in this field, and it would help them to develop a new eco-friendly, efficient and economical method. This is necessary from today's point of view as we need an environmentally clean protocol for the large scale production of such an important biological moiety, which may be used further in many reactions to develop a potent pharmacophore for the future.Acknowledgements
The authors are thankful to the Department of Chemistry, Gujarat University, Ahmedabad, for providing the necessary facilities and also thankful to UGC-Info net & INFLIBNET Gujarat University for providing e-source facilities.References
- V. V. Kouznetsov, L. Y. Mendez and C. M. Gomez, Curr. Org. Chem., 2005, 9, 141–161 CrossRef CAS.
- R. Heusch and B. Leverkusen, Ullmann's Encyclopedia of Industrial Chemistry, 2000, DOI:10.14356007: a09_297.
- U. Desai, S. Mitragotri, T. Thopate, D. Pore and P. Wadgaonkarb, ARKIVOC, 2006, 198–204 Search PubMed.
- E. E. Ebenso, M. M. Kabanda, T. Arslan, M. Saracoglu, F. Kandemirli, L. C. Murulana, A. K. Singh, S. K. Shukla, B. Hammouti and K. Khaled, Int. J. Electrochem. Sci., 2012, 7, 5643–5676 CAS.
- S. Bawa, S. Kumar, S. Drabu and R. Kumar, J. Pharm. BioAllied Sci., 2010, 2, 64–71 CrossRef CAS PubMed.
- M. ozyanik, S. Demirci, H. Bektas, N. Demirbas, A. Demirbas and S. A. Karaoglu, Turk. J. Chem., 2012, 36, 233–246 CAS.
- P. R. Graves, J. J. Kwiek, P. Fadden, R. Ray, K. Hardeman, A. M. Coley, M. Foley and T. A. Haystead, Mol. Pharmacol., 2002, 62, 1364–1372 CrossRef CAS.
- A. T. Vu, S. T. Cohn, E. S. Manas, H. A. Harris and R. E. Mewshaw, Bioorg. Med. Chem. Lett., 2005, 15, 4520–4525 CrossRef CAS PubMed.
- C. H. Kaschula, T. J. Egan, R. Hunter, N. Basilico, S. Parapini, D. Taramelli, E. Pasini and D. Monti, J. Med. Chem., 2002, 45, 3531–3539 CrossRef CAS PubMed.
- Y. Tokoro, A. Nagai, K. Kokado and Y. Chujo, Macromolecules, 2009, 42, 2988–2993 CrossRef CAS.
- G. Jégou and S. A. Jenekhe, Macromolecules, 2001, 34, 7926–7928 CrossRef.
- Y. Li, X. Shi, N. Xie, Y. Zhao and S. Li, MedChemComm, 2013, 4, 367 RSC.
- S. Vandekerckhove, S. De Moor, D. Segers, C. de Kock, P. J. Smith, K. Chibale, N. De Kimpe and M. D'Hooghe, MedChemComm, 2013, 4, 724 RSC.
- H. R. Bhat, U. P. Singh, P. Gahtori, S. K. Ghosh, K. Gogoi, A. Prakash and R. K. Singh, RSC Adv., 2013, 3, 2942 RSC.
- H. R. Bhat, S. K. Gupta and U. P. Singh, RSC Adv., 2012, 2, 12690 RSC.
- J. McNulty, R. Vemula, C. Bordon, R. Yolken and L. Jones-Brando, Org. Biomol. Chem., 2014, 12, 255–260 CAS.
- S. Gogoi, K. Shekarrao, A. Duarah, T. C. Bora and R. C. Boruah, Steroids, 2012, 77, 1438–1445 CrossRef CAS PubMed.
- R. Kwong, J. Am. Chem. Soc., 2005, 127, 1614–1615 CrossRef PubMed.
- H. Tong, L. Wang, X. Jing and F. Wang, Macromolecules, 2003, 36, 2584–2586 CrossRef CAS.
- G. E. Tumambac, C. M. Rosencrance and C. Wolf, Tetrahedron, 2004, 60, 11293–11297 CrossRef CAS PubMed.
- T. R. Reddy, L. S. Reddy, G. R. Reddy, K. Yarbagi, Y. Lingappa, D. Rambabu, G. R. Krishna, C. M. Reddy, K. S. Kumar and M. Pal, Green Chem., 2012, 14, 1870–1872 RSC.
- J. Safari, S. H. Banitaba and S. S. Samiei, J. Chem. Sci., 2009, 121, 481–484 CrossRef CAS PubMed.
- S. Anvar, I. Mohammadpoor-Baltork, S. Tangestaninejad, M. Moghadam, V. Mirkhani, A. R. Khosropour and R. Kia, RSC Adv., 2012, 2, 8713–8720 RSC.
- H. Zhu, R. F. Yang, L. H. Yun and J. Li, Chin. Chem. Lett., 2010, 21, 35–38 CrossRef CAS PubMed.
- B. C. Ranu, A. Hajra and U. Jana, Tetrahedron Lett., 2000, 41, 531–533 CrossRef CAS.
- A. Kulkarni and B. Torok, Green Chem., 2010, 12, 875–878 RSC.
- C. Praveen, P. DheenKumar, D. Muralidharan and P. T. Perumal, Bioorg. Med. Chem. Lett., 2010, 20, 7292–7296 CrossRef CAS PubMed.
- H. R. Prakash Naik, H. S. Bhojya Naik, T. R. Ravikumar Naik, T. Aravinda and D. S. Lamani, Phosphorus, Sulfur Silicon Relat. Elem., 2009, 184, 2109–2114 CrossRef.
- K. Arya and M. Agarwal, Bioorg. Med. Chem. Lett., 2007, 17, 86–93 CrossRef CAS PubMed.
- J. H. Lange, P. C. Verveer, S. J. Osnabrug and G. M. Visser, Tetrahedron Lett., 2001, 42, 1367–1369 CrossRef CAS.
- J. Jampilek, R. Musiol, M. Pesko, K. Kralova, M. Vejsova, J. Carroll, A. Coffey, J. Finster, D. Tabak and H. Niedbala, Molecules, 2009, 14, 1145–1159 CrossRef CAS PubMed.
- G. Nagendrappa, Appl. Clay Sci., 2011, 53, 106–138 CrossRef CAS PubMed.
- M. Jida and B. Deprez, New J. Chem., 2012, 36, 869 RSC.
- J. Wu, H. G. Xia and K. Gao, J. Org. Biomol. Chem., 2006, 4, 126–129 RSC.
- M. A. Zolfigol, P. Salehi, A. Ghaderi and M. Shiri, J. Chin. Biochem. Soc., 2007, 54, 267–271 CAS.
- M. Hosseini-Sarvari, J. Iran. Chem. Soc., 2011, 8, 119–128 CrossRef.
- M. R. Heravi, Ultrason. Sonochem., 2009, 16, 361–366 CrossRef CAS PubMed.
- T. A. Bryson, J. M. Gibson, J. J. Stewart, H. Voegtle, A. Tiwari, J. H. Dawson, W. Marley and B. Harmon, Green Chem., 2003, 5, 177–180 RSC.
- A. Hasaninejad, M. Shekouhy and A. Zare, Catal. Sci. Technol., 2012, 2, 201–214 CAS.
- A. Teimouri and A. Najafi Chermahini, Arabian J. Chem., 2011 DOI:10.1016/j.arabjc.2011.05.018.
- C. S. Cho and W. X. Ren, J. Organomet. Chem., 2007, 692, 4182–4186 CrossRef CAS PubMed.
- M. A. Zolfigol, P. Salehi, A. Ghaderi, M. Shiri and Z. Tanbakouchian, J. Mol. Catal. A: Chem., 2006, 259, 253–258 CrossRef CAS PubMed.
- X. Li, Z. Mao, Y. Wang, W. Chen and X. Lin, Tetrahedron, 2011, 67, 3858–3862 CrossRef CAS PubMed.
- J. S. Yadav, P. Purushothama Rao, D. Sreenu, R. S. Rao, V. Naveen Kumar, K. Nagaiah and A. R. Prasad, Tetrahedron Lett., 2005, 46, 7249–7253 CrossRef CAS PubMed.
- A. H. Li, E. Ahmed, X. Chen, M. Cox, A. P. Crew, H. Q. Dong, M. Jin, L. Ma, B. Panicker, K. W. Siu, A. G. Steinig, K. M. Stolz, P. A. Tavares, B. Volk, Q. Weng, D. Werner and M. J. Mulvihill, Org. Biomol. Chem., 2007, 5, 61–64 CAS.
- K. Motokura, T. Mizugaki, K. Ebitani and K. Kaneda, Tetrahedron Lett., 2004, 45, 6029–6032 CrossRef CAS PubMed.
- B. Mirza and S. S. Samiei, J. Chem. Chem. Eng., 2011, 5, 644–647 CAS.
- M. K. Chaudhuri and S. Hussain, J. Chem. Sci., 2006, 118, 199–202 CrossRef CAS.
- C. Yao, B. Qin, H. Zhang, J. Lu, D. Wang and S. Tu, RSC Adv., 2012, 2, 3759 RSC.
- H. Huang, H. Jiang, K. Chen and H. Liu, J. Org. Chem., 2009, 74, 5476–5480 CrossRef CAS PubMed.
- E. Kowsari and M. Mallakmohammadi, Ultrason. Sonochem., 2011, 18, 447–454 CrossRef CAS PubMed.
- M. Austin, O. J. Egan, R. Tully and A. C. Pratt, Org. Biomol. Chem., 2007, 5, 3778–3786 CAS.
- T. Mitamura and A. Ogawa, J. Org. Chem., 2011, 76, 1163–1166 CrossRef CAS PubMed.
- K. H. Park, H. S. Joo, K. I. Ahn and K. Jun, Tetrahedron Lett., 1995, 36, 5943–5946 CrossRef CAS.
- K. Selvam and M. Swaminathan, RSC Adv., 2012, 2, 2848–2855 RSC.
- K. Selvam and M. Swaminathan, Tetrahedron Lett., 2010, 51, 4911–4914 CrossRef CAS PubMed.
- K. Selvam and M. Swaminathan, J. Mol. Catal. A: Chem., 2011, 351, 52–61 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |