Stereochemistry and miscibility of epoxy resin–poly(trimethylene terephthalate) blends
Abstract
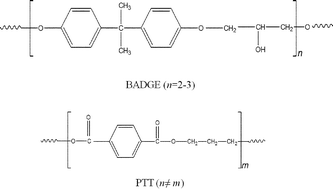
Stereochemistry is proposed to contribute to the miscibility of poly(trimethylene terephthalate) (PTT) and bisphenol-A diglycidyl ether (BADGE), since molecular conformation is one of the determinants of the close packing ability and hence the interactions of such a system. Polymer blends were prepared by the conventional melt mixing technique using a HAAKE mixer. The influence of the stereochemistries of the mixing polymers on the miscibility has been poorly investigated in the literature. The amorphous fraction of PTT was found to be miscible with BADGE, while the crystalline fraction was found to be immiscible with BADGE. This is an interesting situation where one fraction of the polymer is miscible while another fraction is immiscible. The stereochemistries of the glycol residues of PTT are different in the crystalline and the amorphous fractions, and therefore the miscibility of the amorphous fraction of PTT with BADGE could be due to the π–π and n–π interactions between the phenyl and carbonyl groups of PTT with the aromatic rings of BADGE. Fourier transform infra-red microscopy (FTIR microscopy), wide angle X-ray scattering (WAXS) and thermogravimetric analyses show that PTT and BADGE retain their identities in the blend, eliminating the possibility that any trans reactions occur between the blend components.

 Please wait while we load your content...
Please wait while we load your content...