Effect of carbon type on the performance of a direct or hybrid carbon solid oxide fuel cell
Abstract
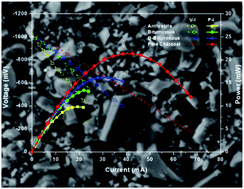
The impact of carbon type on the performance of the direct carbon fuel cell (DCFC) or hybrid carbon fuel cell (HCFC) is investigated by utilizing bare carbon or carbon/carbonate mixtures as feedstock, respectively. In this regard, four different types of carbons, i.e. bituminous coal (BC), demineralised bituminous coal (DBC), anthracite coal (AC) and pine charcoal (PCC), are employed as fuels in a SOFC of the type: carbon (carbonate)|Cu–CeO2/YSZ/Ag|Air. The results reveal that in the absence of carbonates (DCFC configuration) the optimum performance, in terms of maximum power density (Pmax), is obtained for the charcoal sample, which demonstrated a power output of ∼12 mW cm−2 at 800 °C, compared to 3.4 and 4.6 mW cm−2 with the anthracite and bituminous samples, respectively. Demineralization treatment of bituminous coal is found to improve the DCFC performance resulting in a maximum power density of 5.5 mW cm−2. A similar trend in terms of maximum power density, i.e., PCC > DBC > BC > AC, is obtained in the hybrid carbon fuel cell (HCFC) employing a eutectic mixture of lithium and potassium carbonates (62 mol% Li2CO3 + 38 mol% K2CO3) in the anode compartment at a carbon/carbonate weight ratio of 4 : 1. An enhancement of up to 185% in the maximum power density is achieved by admixing molten carbonates with carbon feedstock, with its extent being dependent on carbon type and temperature. The obtained results are interpreted on the basis of carbon physicochemical characteristics and their impact on DCFC performance. It is found that the observed trend in volatile matter, porosity and structure disorder is perfectly correlated with the achieved power output. In contrast, high ash and sulfur contents notably inhibit the electrochemical performance. The superior performance demonstrated by pine charcoal in conjunction with its availability and renewable nature, reveals the potential of biomass as feedstock in both DCFCs and HCFCs.
- This article is part of the themed collection: Nanoscience and nanotechnology in electrochemistry

 Please wait while we load your content...
Please wait while we load your content...