Alkaline quaternary ammonium hydroxides and their polymer electrolytes for electrochemical capacitors
Abstract
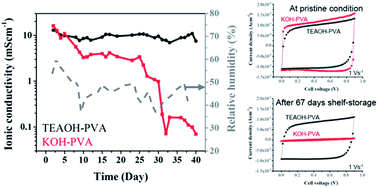
Aqueous quaternary ammonium hydroxides were investigated as electrolytes for electrochemical capacitors (ECs) in both liquid and polymer-gel forms. Alkaline polymer electrolytes, composed of tetraethylammonium hydroxide (TEAOH) and poly(vinyl alcohol) (PVA) in different ratios, were developed and characterized. An optimized TEAOH–PVA electrolyte was developed and compared to KOH–PVA under controlled and ambient conditions. The stability of the polymer electrolytes and the solid ECs were characterized using cyclic voltammetry and electrochemical impedance spectroscopy. While both polymer electrolyte-based ECs demonstrated high rate capability in pristine condition, the TEAOH-based solid EC device showed a much better shelf life than the KOH-based device. This is likely due to the water retention capability of TEAOH, which promoted the amphorous phase and ion mobility, contributing to higher ionic conductivity and better environmental stability.

 Please wait while we load your content...
Please wait while we load your content...