Structure–function study of tertiary amines as switchable polarity solvents
Abstract
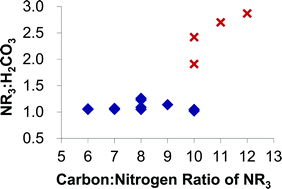
A series of tertiary amines have been screened for their function as switchable polarity solvents (SPS). The relative ratios of tertiary amine and carbonate species as well as maximum possible concentration were determined through quantitative 1H and 13C NMR spectroscopy. The viscosities of the polar SPS solutions were measured and ranged from near water in dilute systems through to gel formation at high concentrations. The van't Hoff indices for SPS solutions were measured through freezing point depression studies as a proxy for osmotic pressures. A new form of SPS with an amine : carbonate ratio significantly greater than unity has been identified. Tertiary amines that function as SPS at ambient pressures appear to be limited to molecules with fewer than 12 carbons. The N,N-dimethyl-n-alkylamine structure has been identified as important to the function of an SPS.

 Please wait while we load your content...
Please wait while we load your content...