Role of basic sites of substituted ferrocenes in interaction with the trinuclear 3,5-bis(trifluoromethyl)pyrazolates: thermodynamics and structure of complexes†
Abstract
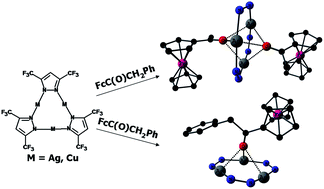
Formation of complexes of the macrocycles (ML)3, where L = 3,5-(CF3)2Pz = 3,5-bis(trifluoromethyl)pyrazolate, M = Cu and Ag, and the acylferrocenes FcC(O)CH2R (Fc = (C5H5)Fe(C5H4); R = H (1), Ph (2)) was studied by means of variable temperature IR, UV-vis, NMR spectroscopy. The sole site of coordination in solution is the oxygen atom of the CO group. The complex composition (1 : 1) and thermodynamic parameters in hexane solution were determined, the formation constants and the enthalpies decreasing from 1 to 2 and from Ag to the Cu macrocycle. The same coordination site featuring triple coordination of oxygen to all metal atoms of a macrocycle was found in the solid state by single crystal X-ray diffraction. There are no shortened contacts of the metal in the macrocycles with π-electron system of the ferrocene's cyclopentadienyl ligands in all complexes. The complexes of (ML)3 with 1 have 1 : 2 composition and bipyramidal structure whereas 2 forms the 1 : 1 complex with (AgL)3. The latter is packed in the infinite stacks involving additional contacts with Ph groups.

 Please wait while we load your content...
Please wait while we load your content...