Multilayer thin film encapsulation for organic light emitting diodes
Abstract
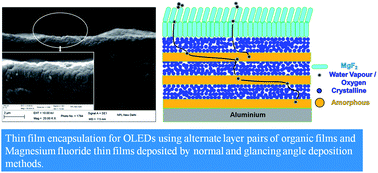
A transparent and effective thin film encapsulation (TFE) based on a multilayer structure is demonstrated. Alternate films of amorphous and crystalline film forming organic materials have been investigated to create complicated diffusion pathways for oxygen and water vapour, which was reflected in their increased barrier properties. These layers are further protected by an inorganic barrier coating of magnesium fluoride (MgF2) deposited by normal and glancing angle deposition methods. A significant enhancement of more than 8 times in the lifetime of organic light emitting diodes was obtained by employing this multilayer hybrid geometry. The TFE structure can be highly useful in organic opto-electronic devices requiring easy to deposit and an effective barrier layer for enhanced lifetimes.

 Please wait while we load your content...
Please wait while we load your content...