Chitosan supported ionic liquid: a recyclable wet and dry catalyst for the direct conversion of aldehydes into nitriles and amides under mild conditions†
Abstract
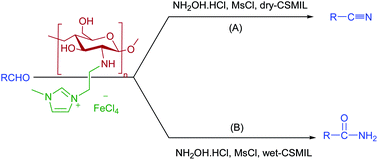
A green and highly efficient chitosan supported magnetic ionic liquid (CSMIL) was synthesized with chitosan (the most abundant biopolymer in nature and a cheap industrial waste product), methyl imidazole and anhydrous/hydrous FeCl3. The heterogeneous catalyst thus obtained was used for the direct conversion of aldehydes to the corresponding nitriles in the presence of NH2OH·HCl/dry-CSMIL/MeSO2Cl and amides with NH2OH·HCl/wet-CSMIL/MeSO2Cl. A highlight of our approach is the easy separation of the catalyst from the reaction medium and thus the recyclability of the catalyst. This simple method can be applied to obtain a wide range of aromatic, heterocyclic, and aliphatic nitriles and amides.

 Please wait while we load your content...
Please wait while we load your content...