Synthesis, structures, and magnetic properties of a series of new heterometallic hexanuclear Co2Ln4 (Ln = Eu, Gd, Tb and Dy) clusters†
Chong-Bin
Tian
a,
Da-Qiang
Yuan
 a,
Yun-Hu
Han
ab,
Zhi-Hua
Li
a,
Ping
Lin
a and
Shao-Wu
Du
*a
a,
Yun-Hu
Han
ab,
Zhi-Hua
Li
a,
Ping
Lin
a and
Shao-Wu
Du
*a
aState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fujian, Fuzhou 350002, P. R. China. E-mail: swdu@fjirsm.ac.cn; Fax: (+86) 591 83709470
bUniversity of Chinese Academy of Sciences, Beijing 100039, P. R. China
First published on 25th September 2014
Abstract
A series of new Co–Ln heterometallic clusters formulated as [Co2Ln4(μ3-OH)2(piv)4(hmmp)4(ae)2]·(NO3)2·2H2O (Ln = Eu (1), Gd (2), Tb (3), Dy (4), H2hmmp = 2-[(2-hydroxyethylimino)methyl]-6-methoxyphenol, Hae = 2-aminoethanol, Hpiv = pivalic acid) were synthesized and characterized. X-ray crystallography reveals that each of them contains a heterometallic {Ln4Co2} core, which is supported by two μ3-hydroxide, four piv−, four hmmp2− and two ae− ligands. The magnetic investigation indicates that 2 exhibits weak antiferromagnetic interactions between GdIII ions, and a large MCE value of 24.9 J kg−1 K−1, while 4 shows a fast relaxation of magnetization. The TGA and VT-PXRD measurements suggest that all the compounds show good thermal stability and can be stable up to about 220 °C. In addition, to address the influence of the Gd–O–Gd angles on the magnetic properties, compound 2 was compared with a series of compounds involving different bridges between GdIII ions. The comparison reveals that the tiny difference in the Gd–O–Gd angles favors different magnetic coupling.
Introduction
The research on nano-sized molecular magnetic materials with a high spin ground state has witnessed in recent years flourishing progress due to their aesthetically fascinating structures and potential applications in many fields such as quantum computing, high-density magnetic information storage, molecular spintronics and low temperature magnetic refrigerant technology.1 One special attractive goal of this research is the synthesis of molecular magnetic materials having the cryogenic magnetocaloric effect (MCE) as candidate materials for magnetic refrigerators.1f,2 The MCE is a magneto-thermodynamic phenomenon that is characterized by the isothermal entropy change (ΔSM) and by the adiabatic temperature change (ΔTad) associated with the magnetic field variation.2,3 Compared with the traditional gas compression–expansion technology, adiabatic demagnetization is less harmful to the environment, more energy efficient, low-lying noise2,3 and much easier to achieve very-low temperatures, even milliKelvin temperatures.4 These virtues make it particularly attractive to scientists, and hence much effort has been invested in research related to materials exhibiting MCE properties.5 Indeed, the MCE is intrinsic in any magnetic material, but only in a few cases is the entropy change sufficiently large to make them suitable for practical use. For example, gadolinium is a suitable candidate for magnetic refrigerant materials mainly because of its 8S7/2 ground state which provides the largest entropy per ion, together with a negligible superexchange interaction. As a result, many Mn–Gd,6 Fe–Gd,7 Co–Gd,8 Ni–Gd,9 Cu–Gd,9b,10 Cr–Gd11 or purely Gd-based5d,12 molecular magnetic cryocooling materials have been reported. However, most of them are coordinated by solvent molecules, which bind to the central metal ions with weaker chemical bonds compared with the coordinated organic ligands, leading to a lower thermal stability. Very recently, Zheng et al. have reported a series of 3d-Gd clusters without coordination solvent molecules by employing phosphonates as ligands,6b,8c,d,9a which display a fascinating large magnetocaloric effect. These clusters are synthesized by a solvothermal method and their thermal stability has not yet been investigated. For a MCE material, the alignment of randomly oriented magnetic moments by an external magnetic field can result in heating, and this heat may lead to damage of the material. Therefore, good thermal stability is important for this type of material.On the other hand, Dy clusters, in particular planar “butterfly” type Dy4 clusters, are of particular interest to the researchers in the field of single-molecular magnets (SMMs) owing to the large magnetic anisotropy and magnetic moment of the Dy component. Over the last few years, a large number of Dy4 butterfly clusters have been reported.13 However, the DyIII ions in these compounds are mainly eight coordinate, and there are only two examples in which the coordination numbers of DyIII ions are greater than eight.13b,c It has been shown that the ligand field as well as the coordination geometry strongly influence the local anisotropy of the DyIII ion, and govern the SMM behaviour.14 Therefore, it is interesting to synthesize new “butterfly” type Dy4 clusters with other coordination geometries and explore their SMM behaviour.
The polydentate hydroxyl-rich Schiff bases, derived from the reactions of 2-hydroxybenzaldehyde or its derivatives with a range of amino alcohols, have been proven to be versatile chelating and bridging ligands which can react with metal ions to afford a number of polynuclear 3d15 or 4f16 clusters. By comparison, less work has been done in the preparation of 3d–4f clusters with these ligands.6d,17 One of these ligands, namely 2-[(2-hydroxyethylimino)methyl]-6-methoxyphenol (H2hmmp), in situ generated from the reaction of 2-hydroxy-3-methoxybenzaldehyde with 2-aminoethanol, has recently been used to make Mn–Ln clusters.18 However, it has not been used in the synthesis of Co–Ln clusters. Herein, we report a series of Co–Ln clusters derived from H2hmmp, formulated as [Co2Ln4(μ3-OH)2(piv)4 (hmmp)4(ae)2]·(NO3)2·2H2O (Ln = Eu 1, Gd 2, Tb 3, Dy 4, Hae = 2-aminoethanol and Hpiv = pivalic acid). The thermal analysis studies show that 1–4 have a high thermal stability up to 220 °C. Magnetic properties of 1–4 are also investigated. Isothermal magnetization measurements indicate that the Co2Gd4 cluster exhibits a large MCE. Moreover, alternating current susceptibility measurements indicate that the Co2Dy4 cluster displays fast quantum tunneling relaxation.
Experimental
Materials and physical measurements
All the materials were purchased from commercial sources and used without further purification. Thermogravimetric experiments were performed using a TGA/NETZSCH STA449C instrument heated from 30 to 800 °C (heating rate 10 °C min−1, nitrogen stream). Elemental analyses of C, H and N were carried out with a Vario EL III elemental analyzer. High-resolution powder XRD patterns were collected using a PANalytical X'Pert Pro (Cu-Kα radiation: λ = 1.54056 Å) in the range of 5° < 2θ < 60°. IR spectra were recorded on a Perkin-Elmer Spectrum One using KBr pellets in the range of 4000–400 cm−1. Magnetic susceptibilities were measured on polycrystalline samples with a Quantum Design PPMS-9 T system. Diamagnetic corrections were made using Pascal's constants.Synthesis of compounds 1–4
The same procedure was employed to prepare all the compounds and hence only the synthesis of 4 is described here in detail. A mixture of 2-hydroxy-3-methoxybenzaldehyde (152 mg, 1.00 mmol) and 2-aminoethanol (76 mg, 1.25 mmol) in MeCN (30 mL) was stirred and heated at 80 °C for one hour and the solution turned dark yellow. After cooling, triethylamine (405 mg, 4.00 mmol), Dy(NO3)3·6H2O (456 mg, 1.00 mmol) and pivalic acid (102 mg, 1.00 mmol) were added to give a light yellow solution. After stirring for another one hour, Co(NO3)2·6H2O (125 mg, 0.50 mmol) and 3-butylene glycol (270 mg, 3.00 mmol) were added and the solution turned dark red. The solution was stirred under ambient conditions for another two hours and then filtered. Orange-yellow crystals of 4 were obtained after one week, washed with cold MeCN (2 × 5 mL) and dried under vacuum (205 mg, yield 36%). Elemental analysis calcd (%) for C64H98N8O32Co2Dy4: C 34.02, H 4.37, N 4.96; Found: C 33.89, H 4.23, N 4.85. IR (KBr, cm−1): 3626 (v), 3413 (v), 3220 (v), 3115 (v), 2959 (m), 2925 (m), 2867 (m), 2695 (vw), 1643 (vw), 1631 (m), 1605 (v), 1563 (s), 1538 (m), 1484 (vw), 1471 (m), 1456 (vw), 1423 (s), 1385 (m), 1325 (vw), 1297 (m), 1223 (s), 1168 (w), 1062 (m), 970 (w), 920 (vw), 894 (vw), 739 (m), 548 (m).Compounds 1–3 were obtained by the same procedures as that described for 4, using Ln(NO3)3·6H2O (Ln = Eu, Gd and Tb) in place of Dy(NO3)3·6H2O. Compound 1: yield: 36%. Elemental analysis calcd (%) for C64H98N8O32Co2Eu4: C 34.67, H 4.46, N 5.05; Found: C 34.72, H 4.49, N 5.11. IR (KBr, cm−1): 3620 (v), 3412 (v), 3219 (v), 3113 (v), 2959 (m), 2928 (m), 2866 (m), 2691 (vw), 1644 (vw), 1630 (s), 1605 (m), 1558 (s), 1532 (m), 1484 (vw), 1456 (vw), 1423 (v), 1384 (v), 1325 (vw), 1297 (m), 1220 (s), 1168 (w), 1060 (s), 968 (w), 916 (vw), 893 (vw), 739 (m), 545 (v). Compound 2: yield: 43%. Elemental analysis calcd (%) for C64H98N8O32Co2Gd4: C 34.34, H 4.41, N 5.01; Found: C 34.46, H 4.48, N 5.10. IR (KBr, cm−1): 3622 (v), 3411 (v), 3222 (v), 3114 (v), 2959 (m), 2927 (m), 2867 (m), 2691 (vw), 1642 (vw), 1631 (s), 1605 (m), 1560 (s), 1535 (m), 1484 (vw), 1456 (vw), 1423 (v), 1385 (v), 1325 (vw), 1297 (m), 1221 (s), 1168 (w), 1061 (s), 969 (w), 917 (vw), 894 (vw), 739 (m), 546 (v). Compound 3: yield: 38%. Elemental analysis calcd (%) for C64H98N8O32Co2 Tb4: C 34.24, H 4.40, N 5.00; Found: C 34.33, H 4.46, N 5.06. IR (KBr, cm−1): 3624 (v), 3412 (v), 3221 (v), 3115 (v), 2959 (m), 2926 (m), 2867 (m), 2695 (vw), 1640 (vw), 1631 (s), 1605 (m), 1561 (s), 1536 (m), 1484 (vw), 1471 (vw), 1423 (v), 1384 (v), 1325 (vw), 1297 (m), 1222 (s), 1168 (w), 1061 (s), 970 (w), 918 (vw), 894 (vw), 740 (m), 547 (v).
X-ray crystallography
Suitable single crystals of the compounds were carefully selected and glued to thin glass fibers with epoxy resin. Intensity data were collected at room temperature on a Rigaku Mercury CCD area-detector diffractometer with a graphite monochromator utilizing Mo-Kα radiation (λ = 0.71073 Å). CrystalClear software19 was used for data reduction and empirical absorption correction. The structures were solved by direct methods using SHELXTL and refined by full-matrix least-squares on F2 using the SHELX-97 program.20 All the non-hydrogen atoms were refined anisotropically, except for O14, C23, C28, C29 and C30 atoms in 1, O14, O15, C23, C28, C29 and C30 atoms in 2, O14, O15, C28, C29 and C30 atoms in 3 and 4. The hydrogen atoms bonded to carbon are generated geometrically (C−H 0.97 or 0.93 Å) and U(H) values are set as 1.2 times Ueq(C). Since the position of the disordered water molecules could not be resolved from the Fourier maps, PLATON/SQUEEZE21 was used to compensate the data for their contribution to the diffraction patterns for 1–4. The final chemical formulae of 1–4 were calculated from SQUEEZE results combined with the TGA and elemental analysis data. Crystallographic data and other pertinent information for these compounds are summarized in Table 1. Selected bond lengths (Å) and angles (°) for compounds 1–4 are listed in Table S1.† CCDC numbers for compounds 1–4 are 948361–948364.| 1 (Eu) | 2 (Gd) | 3 (Tb) | 4 (Dy) | |
|---|---|---|---|---|
| a R 1 = ∑||Fo| − |Fc||/∑|Fo|. b wR2 = [∑w(Fo2 − Fc2)2/∑w(Fo2)2]0.5. | ||||
| Formula | C64H94N8O30Co2Eu4 | C64H94N8O30Co2Gd4 | C64H94N8O30Co2Tb4 | C64H94N8O30Co2Dy4 |
| Formula mass | 2183.53 | 2202.33 | 2209.01 | 2223.33 |
| Crystal system | Triclinic | Triclinic | Triclinic | Triclinic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a/Å | 11.579(5) | 11.628(4) | 11.568(5) | 11.576 (2) |
| b/Å | 13.984(6) | 13.997(4) | 13.976(6) | 14.014 (3) |
| c/Å | 15.517(7) | 15.441(5) | 15.460(7) | 15.380 (3) |
| α/° | 76.066(17) | 74.478(14) | 75.651(15) | 74.549 (9) |
| β/° | 68.290(16) | 68.117(12) | 68.137(14) | 67.993 (9) |
| γ/° | 70.713(18) | 69.735(12) | 70.478(15) | 69.888 (9) |
| V/Å3 | 2183.0(17) | 2159.8(12) | 2164.5(17) | 2144.8 (7) |
| Z | 1 | 1 | 1 | 1 |
| μ/mm−1 | 3.277 | 3.479 | 3.675 | 3.895 |
| D calcd/g cm−3 | 1.659 | 1.693 | 1.695 | 1.727 |
| F(000) | 1080 | 1084 | 1108 | 1092 |
| Reflns measured | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 096 096 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180 180 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 203 203 |
22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 834 834 |
| Independent reflns | 7435 | 9808 | 7584 | 9662 |
| Observed reflns | 6380 | 7955 | 6335 | 7402 |
R
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a [I > 2σ(I)] a [I > 2σ(I)] |
0.0408 | 0.0490 | 0.0475 | 0.0476 |
wR2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b [I > 2σ(I)] b [I > 2σ(I)] |
0.1062 | 0.1304 | 0.1135 | 0.1209 |
| GOF on F2 | 1.096 | 1.077 | 1.099 | 1.012 |
Results and discussion
Syntheses, thermal stability and spectral analysis
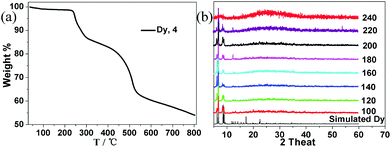
A reaction designed to lead to the in situ formation of a Schiff base as a ligand has been employed to synthesize compounds 1–4. During the reactions, the Schiff base H2hmmp is formed which subsequently reacts with LnIII and CoII ions to generate a series of new CoII–LnIII clusters. Considering large magnetic anisotropy of the CoII ion, our initial aim was to obtain CoII–LnIII clusters. Unfortunately, the oxidation of CoII to CoIII occurred during the reactions, resulting in the presence of CoIII ions in the final products. It should be noted that the addition of 3-butylene glycol can greatly improve the yields of the products. Although a variety of structural types and compositions have been identified so far for Co–Ln clusters,22 to the best of our knowledge, most of them are prepared in CH3OH or CH3OH–CH2Cl2 solution. As a result, one or more CH3OH molecules are coordinated to the metal centres. In the preparation of 1–4, excess 2-aminoethanol was used to prevent the coordination of solvent molecules and we successfully obtained four Co–Ln clusters without any coordinated solvent molecules at the metal centres. Powder X-ray diffraction (PXRD) patterns measured in the solid state have been used to check the phase purity of the bulk samples. The measured PXRD patterns of 1–4 are in good agreement with the simulated ones generated from the results of single-crystal diffraction data, indicating the phase purity of the as-synthesized samples (Fig. S1†). TGA experiments were performed to determine the thermal stability of 1–4 under a nitrogen atmosphere in the temperature range of 30–800 °C. TG analyses indicate that all these compounds have high thermal stability and exhibit similar thermal behaviour (Fig. 1a and S2†). Therefore only the thermal stability of 4 was discussed in detail. The TGA curve of 4 shows a weight loss of 1.54% from 40 to 230 °C, which can be attributed to the loss of two lattice water molecules (calcd = 1.59%). And then, it begins to decompose due to the collapse of organic ligands. The high thermal stability of 1–4 was further confirmed from the temperature resolved XRD patterns, which were performed after calcination of the sample at elevated temperatures in the range of 100–240 °C (Fig. 1b and S2†). The powder XRD patterns of 4 fit well with the simulated data below 220 °C, which indicate that it remains crystalline below this temperature (Fig. 1b). At 240 °C, the long-range order of the structure was lost and the amorphous phase was formed. The thermal stability of 4 is comparable to metal–organic frameworks (MOFs) that generally decompose in the temperature range of 200–350 °C.23 As shown in Fig. S3,† the IR spectra of these compounds are nearly identical. The absorption bands resulting from the skeletal vibrations of the aromatic ring in 1–4 were found in the range of 1400–1600 cm−1. The broad peaks around 3410 cm−1 indicate the presence of free water molecules. The sharp peak around 3625 cm−1 suggests the presence of hydroxyl groups in these compounds. The stretching bands for –NH2 groups appear at about 3220 and 3113 cm−1. Additionally, the IR spectra of 1–4 display absorption bands at about 2960, 2925, 2867 and 1223 cm−1, which can be assigned to the characteristic signals of the But groups. The above vibration bands are consistent with the single-crystal structure analyses.Crystal structure of compounds 1–4
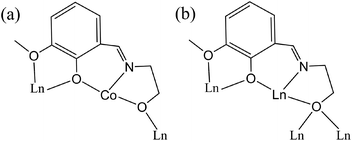
Single crystal X-ray diffraction analysis reveals that 1–4 are isostructural to each other. Therefore, only the description of the Dy analogue will be given here. The asymmetric unit of 4 contains one CoIII ion, two DyIII ions, one OH−, one ae−, two piv−, two hmmp2− and one NO3− anion (Fig. S4†). The Dy1 and Dy2 atoms are both nine-coordinated in a distorted tri-capped trigonal prismatic geometry but with different coordination environments (Fig. 2a): Dy1 is coordinated by one nitrogen atom and eight oxygen atoms with the Dy1–N bond length of 2.49 Å and the average Dy1–O bond length of 2.45 Å, while Dy2 is surrounded by nine oxygen atoms with the average Dy2–O bond length of 2.43 Å. The Co1 atom has a slightly distorted octahedral arrangement with the Co1–LN,O bond lengths ranging from 1.874(6) to 1.942(5) Å. The DyIII ions make up a Dy4O2 core, in which the four DyIII ions exhibit a planar butterfly structure. At the centre of the Dy4 metallic core are two μ3-hydroxide ligands, which bridge the central hinge DyIII (Dy1 and Dy1A) to the outer wing-tip DyIII ions (Dy2 and Dy2A). The two (μ3-OH)− ions are located above and below the Dy4 plane by 0.859 Å (Fig. S5†). Such a Dy4O2 core is bridged to two CoIII ions by two μ3-O atoms (one from an ae− ligand and the other from an hmmp2− ligand), generating a Dy4Co2O12 oxo cluster core (Fig. 2b). Around the periphery of the oxo cluster are two ae−, four piv− and four hmmp2− ligands (Fig. 2c). Both of the ae− ligands adopt a μ3–η1η3 bonding mode. The four piv− ligands are all coordinated to the DyIII ions in the syn–syn and μ–η2 bonding modes, with the former bridging the hinge ions to the wing-tip ions and the latter chelating to the wing-tip ions. The hmmp2− ligands exhibit two bridging modes, μ4–η1η2η1η3 and μ3–η1η2η1η2, to connect DyIII and CoIII ions (Scheme 1). It should be noted that the coordination geometries of DyIII ions in 4 are all tri-capped trigonal prismatic (nine-coordinate) which are different from those of other reported Dy4 butterfly clusters,13,16a where the DyIII ions are mainly in a distorted square-antiprismatic geometry (eight-coordinate).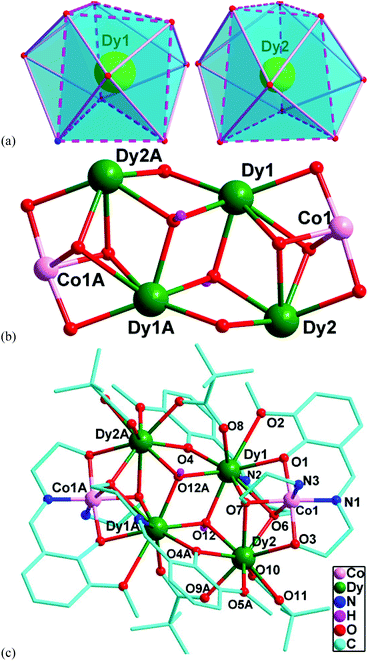 | ||
| Fig. 2 (a) Coordination polyhedron for DyIII ions in 4. (b) The Dy4Co2O12 oxo cluster core in 4. (c) Coordination environments for DyIII and CoIII ions in 4. | ||
Magnetic properties
The variable-temperature dc magnetic susceptibilities for 1–4 were measured in the temperature range of 300–2 K under an applied field of 1000 Oe (Fig. 3a). For 1, the χmT value is 5.27 cm3 mol−1 K at 300 K, which is much larger than the value of 0 cm3 mol−1 K expected for four EuIII ions (J = 0). Due to the presence of the thermally populated excited states of the EuIII ion, the magnetic properties of 1 remain difficult to explain even at room temperature.24 However, its χmT value is close to zero (0.05 cm3 mol−1 K) at 2 K, indicating a nonmagnetic ground state (7F0) for the EuIII ion. This thermally populated diamagnetic ground state of 1 leads to a value of magnetization close to zero even at 8 T (Fig. 3b). Considering the nonmagnetic ground state (7F0) and thermally populated excited states (7FJ, J = 0, 1, 2,…, 6) of the EuIII ion, the magnetic data of 1 can be analyzed using eqn (1) which is deduced from four magnetically non-interacting EuIII ions.25| xm = (Nβ2/3KTx)[24 + (27x/2 + 3/2)e−x + (135x/2 − 5/2)e−3x + (189x − 7/2)e−6x + (405x − 9/2)e−10x + (1485x/2 − 11/2)e−15x + (2457x/2 − 13/2)e−21x]/[1 + 3e−x + 5e−3x + 7e−6x + 9e−10x + 11e−15x + 13e−21x] | (1) |
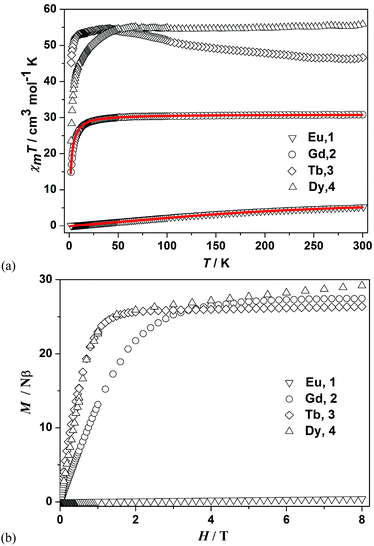 | ||
| Fig. 3 (a) Temperature dependence of the χmT for 1–4 with an applied field of 1000 Oe. The solid line represents the best fit for 1 and 2. (b) Field dependence of magnetization of 1–4 at 2 K. | ||
For 2, the Gd4 displays an χmT value of 30.80 cm3 mol−1 K at room temperature, which is in good agreement with that for four isolated GdIII ions (31.52 cm3 mol−1 K). This value remains constant as the temperature is decreased until ca. 30 K where it sharply decreases to a value of 14.87 cm3 mol−1 K at 2 K. This behaviour is indicative of the occurrence of weak antiferromagnetic interactions between the GdIII ions. In order to quantify the magnitude of the magnetic exchange within the Gd4, the exchange mode with three coupling constants is taken into account (Fig. S6†). The fitting to the magnetic susceptibility curve was performed using the MAGPACK program27 based on the spin-Hamiltonian shown in eqn (2).
| H = −2J1(S1S2 + S3S4) − 2J2(S2S3 + S1S4) − 2J3S1S3 | (2) |
The best fit gave the parameters g = 1.98, J1 = −0.07 cm−1, J2 = −0.05 cm−1 and J3 = −0.029 cm−1 (Fig. 3a). The agreement factor R, defined as Σ[(χmT)obsed − (χmT)calcd]2/Σ(χmT)2obsed, is equal to 2.53 × 10−5. The very small negative J values clearly suggest the weak antiferromagnetic couplings among the GdIII ions. The field dependence of magnetization of 2 shows a saturation value of 27.46Nβ at 2 K and H = 8 T (Fig. 3b), which is close to the expected theoretical value of 28.00Nβ.
The bridges between GdIII ions in 2 can be divided into three types: two μ2-O bridges (type I), three μ2-O bridges (type II) and mixed bridges of one syn–syn carboxylate and two μ2-O bridges (type III) (Fig. S6†). Their values of magnetic coupling are compared with those of other magnetostructurally characterized GdIII complexes (Table 2). It seems that there exists a correlation between the Gd–O–Gd angle (or Gd⋯Gd distance) and the magnetic interaction of GdIII ions. For example, a large Gd–O–Gd angle with type I12c,28 or III12c,29 favours a ferromagnetic interaction. Accordingly, the small Gd–O–Gd angles of types I and III in 2 may lead to an antiferromagnetic interaction between the GdIII ions. It is also found that the type II bridge usually results in antiferromagnetic interactions between the GdIII ions,13d,30 and the smaller the Gd–O–Gd angle, the larger the antiferromagnetic interaction. This observation is in good agreement with the fact that the type II bridge in 2 which has a medium Gd–O–Gd angle shows the presence of a moderate antiferromagnetic interaction.
| Complexa | Gd⋯Gd/Å | Gd–O–Gd/° | J /cm−1 | Bridge | Ref. |
|---|---|---|---|---|---|
a Abbreviations used in this table: OAc = acetate, H2sal = salicylic acid, H2mal = 1,3-propanedioic acid, H4cit = citric acid, Ph2acacH = dibenzoylmethane, acacH = acetylacetone, tpac = 3-thiopheneacetate, pac = pentanoate, H3L = N[(CH2)2N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH-R-CH CH-R-CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–(CH2)2]3N (R = 1,3-(2-OH-5-Me-C6H2)), H3ppa = 6-(3-oxo-3-(2-hydroxyphenyl) propionyl)-2-pyridinecarboxylic acid, py = pyridine, hfac− = 1,1,1,5,5,5-hexafluoroacetylacetonate anion, tpno = tetrathiafulvaleneamido-2-pyridine-N-oxide, H3mta = methanetriacetic acid.
b The spin Hamiltonian is defined as H = −2JSASB. N–(CH2)2]3N (R = 1,3-(2-OH-5-Me-C6H2)), H3ppa = 6-(3-oxo-3-(2-hydroxyphenyl) propionyl)-2-pyridinecarboxylic acid, py = pyridine, hfac− = 1,1,1,5,5,5-hexafluoroacetylacetonate anion, tpno = tetrathiafulvaleneamido-2-pyridine-N-oxide, H3mta = methanetriacetic acid.
b The spin Hamiltonian is defined as H = −2JSASB.
|
|||||
| [Gd2(OAc)6(H2O)4]·4H2O | 4.206 | 115.5 | 0.03 | I | 28a |
| [Gd2(Hsal)6(H2O)2] | 4.250 | 116.12 | 0.025 | I | 28b |
| [Gd2(mal)3(H2O)6]n | 4.276 | 116.71 | 0.024 | I | 28c |
| [Gd(Hcit)(H2O)2]n·nH2O | 4.321 | 118.49 | 0.02 | I | 28d |
| [Gd2(OAc)2(Ph2acac)4(MeOH)2] | 4.128 | 113.65 | 0.019 | I | 12c |
| [Gd2(OAc)6(H2O)4]·2H2O | 4.159 | 115.47 | 0.016 | I | 28e |
| [Gd4(OAc)4(acac)8(H2O)4] | 4.271/4.334 | 114.45/117.73 | 0.012 | I | 12c |
| [Gd2(tpac)6(H2O)4] | 4.126 | 112.5 | −0.006 | I | 28f |
| [Gd2(pac)6(H2O)4] | 4.112 | 113.16 | −0.016 | I | 28f |
| 2 | 3.975 | 112.17 | −0.029 | I | This work |
| [Gd2(L)(NO3)2]·(NO3)·1.5H2O | 3.500 | 92.48/94.93/95.12 | −0.097 | II | 30a |
| [Gd2(Hppa)2(H2ppa)Cl(py)(H2O)] | 3.813 | 98.86/102.93/103.36 | −0.021 | II | 30b |
| [Gd4(μ3-OH)2L2(acac)6]·4CH3CN | 3.689 | 93.52/100.67/102.44 | −0.02 | II | 13d |
| [Gd2(hfac)5(O2CPhCl)(tpno)3]·2H2O | 3.92 | 102.14/103.38/105.37 | −0.005 | II | 30c |
| 2 | 3.77 | 96.28/99.60/104.21 | −0.05 | II | This work |
| [Gd(H2sal)(Hsal)(sal)(H2O)]n | 4.187 | 111.85/114.29 | 0.019 | III | 29a |
| [Gd(OAc)3(MeOH)]n | 4.055 | 110/112.62 | 0.017 | III | 12c |
| [Gd(mta)(H2O)]n·nH2O | 4.065 | 110/112.3 | 0.013 | III | 29b |
| [Gd2(succinate)3(H2O)2]n·0.5nH2O | 4.059 | 109.96/112.24 | 0.01 | III | 29c |
| [Gd(OAc)3(H2O)]n | 4.027/4.034 | 106.5/108.4 | −0.006 | III | 12c |
| 2 | 3.819 | 104.40/105.87 | −0.07 | III | This work |
The χmT products for 3 and 4 display room temperature values of 46.61 and 55.88 cm3 mol−1 K, respectively, which are close to the expected theoretical values using the free ion approximation (47.28 cm3 mol−1 K for 3 and 56.68 cm3 mol−1 K for 4) for four non-interacting lanthanide ions: TbIII (J = 6 and g = 3/2) and DyIII (J = 15/2 and g = 4/3). For 3, the χmT value slowly increases from 46.60 cm3 mol−1 K at 300 K to 54.62 cm3 mol−1 K at about 40 K. Upon further cooling, it decreases to reach 45.16 cm3 mol−1 K at 2 K. The χmT versus T curve shows a broad protuberance at about 40 K, indicating the presence of ferromagnetic interactions between TbIII ions. This kind of interaction is strong enough to compensate the decrease of χmT that resulted from the depopulated Stark states. The ferromagnetic interaction resulting in the increase of χmT upon cooling may be ascribed to the dipole–dipole interaction between TbIII ions.31 For 4, the χmT product remains roughly constant with decreasing temperature down to about 50 K, and then drops to a minimum value of 23.53 cm3 mol−1 K at 2 K. The decrease of χmT in 4 is most likely due to a combination effect of the thermal depopulation of Stark sublevels and antiferromagnetic interactions.32 Due to the presence of the unquenched orbital moment of the TbIII or DyIII center, it is difficult to fit the magnetic data of 3 and 4.
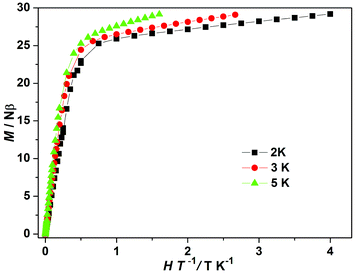
The field-dependent magnetizations for 3 and 4 are nearly the same (Fig. 3b), both showing a regular increase in magnetization below about 1 T. The magnetization of 3 exhibits a plateau at high magnetic field, whereas that of 4 shows a linear increase at high magnetic field without complete saturation even at 8 T. The magnetization values of 26.36Nβ for 3 and 29.19Nβ for 4 at 8 T are lower than their theoretically derived values (36Nβ for 3 and 40Nβ for 4). Furthermore, the M versus H/T curves at low temperatures for 3 and 4 are not superposed (Fig. 4 and S7c†), indicating the presence of magnetic anisotropy. The divergence of M versus H/T curves of 4 is more apparent than that of 3, suggesting that 4 displays a more significant magnetic anisotropy.33
In view of the weak interactions as well as the isotropic nature of GdIII ions in 2, the MCE of 2 is investigated according to the isothermal magnetization curves measured in an applied field of up to 8 T and the temperature range of 2–7 K (Fig. 5a). The magnetic entropy changes −ΔSM can be described by the Maxwell equation as follows:3
| −ΔSm(T)ΔH = ∫[∂M(T, H)/∂T]HdH | (3) |
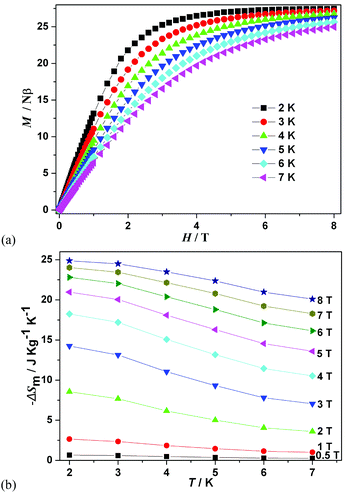 | ||
| Fig. 5 (a) The field dependence of magnetization of 2 at 2–7 K. (b) Calculated −ΔSM using the magnetization data of 2 at various fields (0.5–8 T). | ||
Using eqn (3), magnetic entropy changes ΔSm were calculated from the experimental magnetization data (Fig. 5b). It can be seen that the maximum value of −ΔSm is 24.9 J kg−1 K−1 for ΔH = 8 T at 2 K, which is lower than the expected value of 30.90 J kg−1 K−1 according to the equation nR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(2S + 1) for four isolated GdIII ions. This deviation may be caused by the weak intra-cluster anti-ferromagnetic interactions in 2.7,34
ln(2S + 1) for four isolated GdIII ions. This deviation may be caused by the weak intra-cluster anti-ferromagnetic interactions in 2.7,34
Among the 3d-Gd based magnetic cryocooling materials, a magnetic entropy change −ΔSm larger than 24 J kg−1 K−1 is relatively rare (Table 3). The maximum value of −ΔSm in 2 is larger than most of the reported Co–Gd clusters. Nevertheless, it is surpassed only by four known Co–Gd clusters, {Co10Gd42},8a {Co4Gd10},8b {Co6Gd8}8c and {Co16Gd24},8e with the −ΔSm values of 41.3, 32.6, 28.6 and 28.0 J kg−1 K−1 for ΔH = 7 T, respectively. This is not surprising as 2 contains diamagnetic CoIII ions that reduce the magnetic density, resulting in a low magnetic entropy change.
| Compounds | −ΔSM/J kg−1 K−1 | Ref. |
|---|---|---|
| Co10Gd42 | 41.3 (7 T) | 8a |
| Ni10Gd42 | 38.2 (7 T) | 8a |
| Ni12Gd36 | 36.3 (7 T) | 9d |
| Mn4Gd6 | 33.7 (7 T) | 6b |
| Co4Gd10 | 32.6 (7 T) | 8b |
| Cu5Gd4 | 31.0 (9 T) | 10a |
| Co6Gd8 | 28.6 (7 T) | 8c |
| Mn9Gd9 | 28.0 (7 T) | 6b |
| Fe5Gd8 | 26.7 (7 T) | 7 |
| Ni6Gd6 | 26.5 (7 T) | 9a |
| Co16Gd24 | 26.0 (7 T) | 8e |
| 2 | 24.9 (8 T) | This work |
| Co4Gd6 | 23.6 (7 T) | 9b |
| Cu6Gd6 | 23.5 (7 T) | 10c |
| Co8Gd4 | 22.3 (7 T) | 8b |
| Ni8Gd4 | 22.0 (7 T) | 9b |
| Ni12Gd5 | 21.8 (7 T) | 9e |
| Co8Gd8 | 21.4 (7 T) | 8c |
| Co8Gd4 | 21.1 (7 T) | 9b |
| Cu36Gd24 | 21.0 (7 T) | 10b |
| Zn8Gd4 | 20.8 (7 T) | 9b |
| Co8Gd8 | 20.4 (7 T) | 8d |
| Co4Gd2 | 20.0 (7 T) | 8c |
| Co4Gd6 | 19.9 (7 T) | 8d |
| Co6Gd4 | 19.7 (7 T) | 8b |
| Mn4Gd4 | 19.0 (7 T) | 6a |
| Ni6Gd2 | 17.6 (7 T) | 9c |
| Mn12Gd6 | 17.0 (7 T) | 6d |
| Cu8Gd4 | 14.6 (7 T) | 9b |
| Cu8Gd2 | 12.8 (7 T) | 10d |
| Co8Gd2 | 11.8 (7 T) | 8c |
| Cr2Gd2 | 11.4 (9 T) | 11b |
| Cr7Gd | 5.1 (7 T) | 11a |
Due to the presence of significant magnetic anisotropy in 4, both the temperature and frequency dependences of ac magnetic susceptibilities were performed under a zero dc field (Fig. 6 and S8†). As illustrated in Fig. 6 and S8,† a frequency dependence of the ac susceptibility was observed with an out-of-phase signal that is weak in intensity and does not exhibit a maximum within the experimental temperature (above 2 K) and frequency (50 to 104 Hz) windows, which indicates the presence of fast relaxation via quantum tunneling (QTM) as observed in other low symmetry lanthanide based-SMMs.22j Hence, in order to bypass any possible quantum tunneling effects, ac susceptibility measurements were further performed under applied dc fields of 5000 and 8000 Oe (Fig. S9†). It is found that 4 shows peaks in the temperature dependence of x′′m under applied dc fields, which is due to the suppression of the quantum tunneling of magnetization. The relaxation follows a thermally activated mechanism, giving energy barriers of ΔE/KB = 25.2 and 32.4 K, relaxation times τ0 = 1.3 × 10−6 and 4.2 × 10−7 s at 5000 and 8000 Oe, respectively, based on the Arrhenius law τ(Tp) = τ0exp(Δ/Tp) with a linear correlation of 1/Tpvs. ln(2πf) (Fig. S10†). These energy barriers are comparable to those reported for other compounds exhibiting the field induced SMM behaviour.13d,35 It should be noted that the fast quantum tunneling relaxation observed in the case of 4 may be attributed to the low symmetric coordination geometry of the local DyIII sites as well as the weak intramolecular interactions between the adjacent DyIII ions.36,37
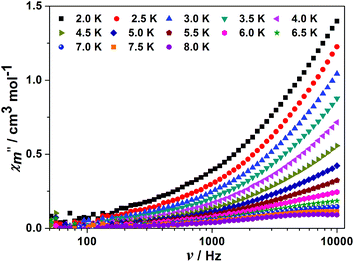 | ||
| Fig. 6 Frequency dependence in the zero dc field of the (x′′m) ac susceptibility component at different temperatures for 4. | ||
Conclusions
In summary, using the in situ synthetic route, we have synthesized a series of new Co–Ln heterometallic clusters, in which the {Co2Ln4} core is bridged by two μ3-hydroxide, four piv−, four hmmp2− and two ae− ligands. Due to the absence of any coordinated solvent molecules at the metal centres, all these compounds have a high thermal stability and can be stable up to 220 °C. The magnetic measurements show that the Co2Gd4 cluster exhibits very weak antiferromagnetic interactions among the GdIII ions, as well as a large magnetocaloric effect at low temperatures, showing a potential application in magnetic cooling technology in the very-low temperature range. Replacement of GdIII ions with anisotropic DyIII ions gives the Dy4Co2 cluster, which displays significantly fast QTM in a zero dc field that can be slowed down by application of an external dc field. Moreover, the magnetostructural study reveals that the tiny change in Gd–O–Gd angles will result in different magnetic interactions. This work will be helpful for the rational design and synthesis of highly thermally stable 3d–4f based molecular magnetic cryogenic materials.Acknowledgements
We thank the National Basic Research Program of China (973 program, 2012CB821702), the National Natural Science Foundation of China (21233009 and 21173221) and the State Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences for financial support.Notes and references
- (a) M. N. Leuenberger and D. Loss, Nature, 2001, 410, 789–793 CrossRef CAS PubMed; (b) S. Hill, R. S. Edwards, N. Aliaga-Alcalde and G. Christou, Science, 2003, 302, 1015–1018 CrossRef CAS PubMed; (c) L. Bogani and W. Wernsdorfer, Nat. Mater., 2008, 7, 179–186 CrossRef CAS PubMed; (d) M. Mannini, F. Pineider, C. Danieli, F. Totti, L. Sorace, P. Sainctavit, M. A. Arrio, E. Otero, L. Joly, J. C. Cezar, A. Cornia and R. Sessoli, Nature, 2010, 468, 417–421 CrossRef CAS PubMed; (e) G. E. Kostakis, S. P. Perlepes, V. A. Blatov, D. M. Proserpio and A. K. Powell, Coord. Chem. Rev., 2012, 256, 1246–1278 CrossRef CAS; (f) B. G. Shen, J. R. Sun, F. X. Hu, H. W. Zhang and Z. H. Cheng, Adv. Mater., 2009, 21, 4545–4564 CrossRef CAS.
- R. Sessoli, Angew. Chem., Int. Ed., 2012, 51, 43–45 CrossRef CAS PubMed.
- (a) M. Evangelisti, F. Luis, L. J. de Jongh and M. Affronte, J. Mater. Chem., 2006, 16, 2534–2549 RSC; (b) M. Evangelisti and E. K. Brechin, Dalton Trans., 2010, 39, 4672–4676 RSC.
- M.-J. Martinez-Perez, O. Montero, M. Evangelisti, F. Luis, J. Sese, S. Cardona-Serra and E. Coronado, Adv. Mater., 2012, 24, 4301–4305 CrossRef CAS PubMed.
- (a) F.-S. Guo, Y.-C. Chen, J.-L. Liu, J.-D. Leng, Z.-S. Meng, P. Vrabel, M. Orendac and M.-L. Tong, Chem. Commun., 2012, 48, 12219–12221 RSC; (b) G. Lorusso, M. A. Palacios, G. S. Nichol, E. K. Brechin, O. Roubeau and M. Evangelisti, Chem. Commun., 2012, 48, 7592–7594 RSC; (c) R. Sibille, T. Mazet, B. Malaman and M. Francois, Chem. – Eur. J., 2012, 18, 12970–12973 CrossRef CAS PubMed; (d) L.-X. Chang, G. Xiong, L. Wang, P. Cheng and B. Zhao, Chem. Commun., 2013, 49, 1055–1057 RSC; (e) C.-B. Tian, Z.-J. Lin and S.-W. Du, Cryst. Growth Des., 2013, 13, 3746–3753 CrossRef CAS; (f) C.-B. Tian, R.-P. Chen, C. He, W.-J. Li, Q. Wei, X.-D. Zhang and S.-W. Du, Chem. Commun., 2014, 50, 1915–1917 RSC.
- (a) G. Karotsis, M. Evangelisti, S. J. Dalgarno and E. K. Brechin, Angew. Chem., Int. Ed., 2009, 48, 9928–9931 CrossRef CAS PubMed; (b) Y.-Z. Zheng, E. M. Pineda, M. Helliwell and R. E. P. Winpenny, Chem. – Eur. J., 2012, 18, 4161–4165 CrossRef CAS PubMed; (c) G. Karotsis, S. Kennedy, S. J. Teat, C. M. Beavers, D. A. Fowler, J. J. Morales, M. Evangelisti, S. J. Dalgarno and E. K. Brechin, J. Am. Chem. Soc., 2010, 132, 12983–12990 CrossRef CAS PubMed; (d) J.-L. Liu, W.-Q. Lin, Y.-C. Chen, J.-D. Leng, F.-S. Guo and M.-L. Tong, Inorg. Chem., 2013, 52, 457–463 CrossRef CAS PubMed.
- E. Cremades, S. Gomez-Coca, D. Aravena, S. Alvarez and E. Ruiz, J. Am. Chem. Soc., 2012, 134, 10532–10542 CrossRef CAS PubMed.
- (a) J.-B. Peng, Q.-C. Zhang, X.-J. Kong, Y.-Z. Zheng, Y.-P. Ren, L.-S. Long, R.-B. Huang, L.-S. Zheng and Z. Zheng, J. Am. Chem. Soc., 2012, 134, 3314–3317 CrossRef CAS PubMed; (b) E. M. Pineda, F. Tuna, R. G. Pritchard, A. C. Regan, R. E. P. Winpenny and E. J. L. McInnes, Chem. Commun., 2013, 49, 3522–3524 RSC; (c) Y.-Z. Zheng, M. Evangelisti, F. Tuna and R. E. P. Winpenny, J. Am. Chem. Soc., 2012, 134, 1057–1065 CrossRef CAS PubMed; (d) Y.-Z. Zheng, M. Evangelisti and R. E. P. Winpenny, Chem. Sci., 2011, 2, 99–102 RSC; (e) Z. M. Zhang, L. Y. Pan, W. Q. Lin, J. D. Leng, F. S. Guo, Y. C. Chen, J. L. Liu and M. L. Tong, Chem. Commun., 2013, 49, 8081–8083 RSC.
- (a) Y.-Z. Zheng, M. Evangelisti and R. E. P. Winpenny, Angew. Chem., Int. Ed., 2011, 50, 3692–3695 CrossRef CAS PubMed; (b) T. N. Hooper, J. Schnack, S. Piligkos, M. Evangelisti and E. K. Brechin, Angew. Chem., Int. Ed., 2012, 51, 4633–4636 CrossRef CAS PubMed; (c) A. Hosoi, Y. Yukawa, S. Igarashi, S. J. Teat, O. Roubeau, M. Evangelisti, E. Cremades, E. Ruiz, L. A. Barrios and G. Aromi, Chem. – Eur. J., 2011, 17, 8264–8268 CrossRef CAS PubMed; (d) J.-B. Peng, Q.-C. Zhang, X.-J. Kong, Y.-P. Ren, L.-S. Long, R.-B. Huang, L.-S. Zheng and Z. Zheng, Angew. Chem., Int. Ed., 2011, 50, 10649–10652 CrossRef CAS PubMed; (e) Z.-Y. Li, J. Zhu, X.-Q. Wang, J. Ni, J.-J. Zhang, S.-Q. Liu and C.-Y. Duan, Dalton Trans., 2013, 42, 5711–5717 RSC.
- (a) S. K. Langley, N. F. Chilton, B. Moubaraki, T. Hooper, E. K. Brechin, M. Evangelisti and K. S. Murray, Chem. Sci., 2011, 2, 1166–1169 RSC; (b) J.-D. Leng, J.-L. Liu and M.-L. Tong, Chem. Commun., 2012, 48, 5286–5288 RSC; (c) A. S. Dinca, A. Ghirri, A. M. Madalan, M. Affronte and M. Andruh, Inorg. Chem., 2012, 51, 3935–3937 CrossRef CAS PubMed; (d) H. Zhang, G.-L. Zhuang, X.-J. Kong, Y.-P. Ren, L.-S. Long, R.-B. Huang and L.-S. Zheng, Cryst. Growth Des., 2013, 13, 2493–2498 CrossRef CAS.
- (a) M. Affronte, A. Ghirri, S. Carretta, G. Amoretti, S. Piligkos, G. A. Timco and R. E. P. Winpenny, Appl. Phys. Lett., 2004, 84, 3468–3470 CrossRef CAS; (b) C. A. Thuesen, K. S. Pedersen, M. Schau-Magnussen, M. Evangelisti, J. Vibenholt, S. Piligkos, H. Weihe and J. Bendix, Dalton Trans., 2012, 41, 11284–11292 RSC.
- (a) M. Evangelisti, O. Roubeau, E. Palacios, A. Camon, T. N. Hooper, E. K. Brechin and J. J. Alonso, Angew. Chem., Int. Ed., 2011, 50, 6606–6609 CrossRef CAS PubMed; (b) J. W. Sharples, Y.-Z. Zheng, F. Tuna, E. J. L. McInnes and D. Collison, Chem. Commun., 2011, 47, 7650–7652 RSC; (c) F.-S. Guo, J.-D. Leng, J.-L. Liu, Z.-S. Meng and M.-L. Tong, Inorg. Chem., 2012, 51, 405–413 CrossRef CAS PubMed.
- (a) P.-H. Lin, T. J. Burchell, L. Ungur, L. F. Chibotaru, W. Wernsdorfer and M. Murugesu, Angew. Chem., Int. Ed., 2009, 48, 9489–9492 CrossRef CAS PubMed; (b) S. Xue, L. Zhao, Y.-N. Guo, R. Deng, Y. Go and J. Tang, Dalton Trans., 2011, 40, 8347–8352 RSC; (c) S. K. Langley, N. F. Chilton, I. A. Gass, B. Moubaraki and K. S. Murray, Dalton Trans., 2011, 40, 12656–12659 RSC; (d) P.-F. Yan, P.-H. Lin, F. Habib, T. Aharen, M. Murugesu, Z.-P. Deng, G.-M. Li and W.-B. Sun, Inorg. Chem., 2011, 50, 7059–7065 CrossRef CAS PubMed; (e) G. Abbas, Y. Lan, G. E. Kostakis, W. Wernsdorfer, C. E. Anson and A. K. Powell, Inorg. Chem., 2010, 49, 8067–8072 CrossRef CAS PubMed; (f) P.-H. Guo, J.-L. Liu, Z.-M. Zhang, L. Ungur, L. F. Chibotaru, J.-D. Leng, F.-S. Guo and M.-L. Tong, Inorg. Chem., 2012, 51, 1233–1235 CrossRef CAS PubMed; (g) A. K. Jami, V. Baskar and E. Carolina Sanudo, Inorg. Chem., 2013, 52, 2432–2438 CrossRef CAS PubMed.
- (a) P. Zhang, Y.-N. Guo and J. Tang, Coord. Chem. Rev., 2013, 257, 1728–1763 CrossRef CAS; (b) J. D. Rinehart and J. R. Long, Chem. Sci., 2011, 2, 2078–2085 RSC; (c) D. N. Woodruff, R. E. P. Winpenny and R. A. Layfield, Chem. Rev., 2013, 113, 5110–5148 CrossRef CAS PubMed.
- (a) C.-M. Liu, R.-G. Xiong, D.-Q. Zhang and D.-B. Zhu, J. Am. Chem. Soc., 2010, 132, 4044–4045 CrossRef CAS PubMed; (b) S. Nayak, H. P. Nayek, S. Dehnen, A. K. Powell and J. Reedijk, Dalton Trans., 2011, 40, 2699–2702 RSC; (c) P.-P. Yang, X.-Y. Song, R.-N. Liu, L.-C. Li and D.-Z. Liao, Dalton Trans., 2010, 39, 6285–6294 RSC; (d) I. J. Hewitt, J.-K. Tang, N. T. Madhu, R. Clerac, G. Buth, C. E. Anson and A. K. Powell, Chem. Commun., 2006, 2650–2652 RSC; (e) H. Oshio, N. Hoshino, T. Ito, M. Nakano, F. Renz and P. Gutlich, Angew. Chem., Int. Ed., 2003, 42, 223–225 CrossRef CAS PubMed; (f) L.-L. Fan, F.-S. Guo, L. Yun, Z.-J. Lin, R. Herchel, J.-D. Leng, Y.-C. Ou and M.-L. Tong, Dalton Trans., 2010, 39, 1771–1780 RSC; (g) R. Koner, S. Hazra, M. Fleck, A. Jana, C. R. Lucas and S. Mohanta, Eur. J. Inorg. Chem., 2009, 4982–4988 CrossRef CAS; (h) D. Liu, Q. Zhou, Y. Chen, F. Yang, Y. Yu, Z. Shi and S. Feng, Dalton Trans., 2010, 39, 5504–5508 RSC; (i) S. S. Tandon, S. D. Bunge, J. Sanchiz and L. K. Thompson, Inorg. Chem., 2012, 51, 3270–3282 CrossRef CAS PubMed.
- (a) Y.-Z. Zheng, Y. Lan, C. E. Anson and A. K. Powell, Inorg. Chem., 2008, 47, 10813–10815 CrossRef CAS PubMed; (b) H. Ke, G.-F. Xu, L. Zhao, J. Tang, X.-Y. Zhang and H.-J. Zhang, Chem. – Eur. J., 2009, 15, 10335–10338 CrossRef CAS PubMed; (c) H. Ke, L. Zhao, G.-F. Xu, Y.-N. Guo, J. Tang, X.-Y. Zhang and H.-J. Zhang, Dalton Trans., 2009, 10609–10613 RSC; (d) G.-F. Xu, P. Gamez, S. J. Teat and J. Tang, Dalton Trans., 2010, 39, 4353–4357 RSC.
- (a) J.-L. Liu, F.-S. Guo, Z.-S. Meng, Y.-Z. Zheng, J.-D. Leng, M.-L. Tong, L. Ungur, L. F. Chibotaru, K. J. Heroux and D. N. Hendrickson, Chem. Sci., 2011, 2, 1268–1272 RSC; (b) G. Wu, I. J. Hewitt, S. Mameri, Y. Lan, R. Clerac, C. E. Anson, S. Qiu and A. K. Powell, Inorg. Chem., 2007, 46, 7229–7231 CrossRef CAS PubMed; (c) S. Nayak, O. Roubeau, S. J. Teat, C. M. Beavers, P. Gamez and J. Reedijk, Inorg. Chem., 2010, 49, 216–221 CrossRef CAS PubMed; (d) V. Chandrasekhar, P. Bag, M. Speldrich, J. van Leusen and P. Kogerler, Inorg. Chem., 2013, 52, 5035–5044 CrossRef CAS PubMed.
- P.-P. Yang, X.-L. Wang, L.-C. Li and D.-Z. Liao, Dalton Trans., 2011, 40, 4155–4161 RSC.
- Crystalclear version 1.36, Molecular Structure Corp. and Rigaku Corp., The Woodlands, TX, and Tokyo, Japan, 2000 Search PubMed.
- G. M. Sheldrick, SHELXL-97 Program for X-ray Crystal Structure Refinement, University of Göttingen, Germany, 1997 Search PubMed.
- A. L. Spek, PLATON-97, University of Utrecht, Utrecht, The Netherlands, 1997 Search PubMed.
- (a) K. C. Mondal, A. Sundt, Y. Lan, G. E. Kostakis, O. Waldmann, L. Ungur, L. F. Chibotaru, C. E. Anson and A. K. Powell, Angew. Chem., Int. Ed., 2012, 51, 7550–7554 CrossRef CAS PubMed; (b) L.-F. Zou, L. Zhao, Y.-N. Guo, G.-M. Yu, Y. Guo, J. Tang and Y.-H. Li, Chem. Commun., 2011, 47, 8659–8661 RSC; (c) M. Orfanoudaki, I. Tamiolakis, M. Siczek, T. Lis, G. S. Armatas, S. A. Pergantis and C. J. Milios, Dalton Trans., 2011, 40, 4793–4796 RSC; (d) H. Xiang, Y. Lan, H.-Y. Li, L. Jiang, T.-B. Lu, C. E. Anson and A. K. Powell, Dalton Trans., 2010, 39, 4737–4739 RSC; (e) V. Chandrasekhar, B. M. Pandian, J. J. Vittal and R. Clerac, Inorg. Chem., 2009, 48, 1148–1157 CrossRef CAS PubMed; (f) H. Ke, L. Zhao, Y. Guo and J. Tang, Dalton Trans., 2012, 41, 9760–9765 RSC; (g) V. Gomez, L. Vendier, M. Corbella and J.-P. Costes, Eur. J. Inorg. Chem., 2011, 53–2656 Search PubMed; (h) V. Gomez, L. Vendier, M. Corbella and J.-P. Costes, Inorg. Chem., 2012, 51, 6396–6440 CrossRef CAS PubMed; (i) V. Chandrasekhar, B. M. Pandian, R. Azhakar, J. J. Vittal and R. Clerac, Inorg. Chem., 2007, 46, 5140–5142 CrossRef CAS PubMed; (j) G.-F. Xu, P. Gamez, J. Tang, R. Clerac, Y.-N. Guo and Y. Guo, Inorg. Chem., 2012, 51, 5693–5698 CrossRef CAS PubMed; (k) J.-P. Costes, L. Vendier and W. Wernsdorfer, Dalton Trans., 2011, 40, 1700–1706 RSC; (l) X.-Q. Zhao, Y. Lan, B. Zhao, P. Cheng, C. E. Anson and A. K. Powell, Dalton Trans., 2010, 39, 4911–4917 RSC; (m) E. K. Brechin, S. G. Harris, S. Parsons and R. E. P. Winpenny, Dalton Trans., 1997, 1665–1666 RSC.
- (a) D.-X. Xue, J.-B. Lin, J.-P. Zhang and X.-M. Chen, CrystEngComm, 2009, 11, 183–188 RSC; (b) H.-L. Jiang, N. Tsumori and Q. Xu, Inorg. Chem., 2010, 49, 10001–10006 CrossRef CAS PubMed.
- J. Long, F. Habib, P.-H. Lin, I. Korobkov, G. Enright, L. Ungur, W. Wernsdorfer, L. F. Chibotaru and M. Murugesu, J. Am. Chem. Soc., 2011, 133, 5319–5328 CrossRef CAS PubMed.
- M. Andruh, E. Bakalbassis, O. Kahn, J. C. Trombe and P. Porcher, Inorg. Chem., 1993, 32, 1616–1622 CrossRef CAS.
- (a) X.-J. Wang, Z.-M. Cen, Q.-L. Ni, X.-F. Jiang, H.-C. Lian, L.-C. Gui, H.-H. Zuo and Z.-Y. Wang, Cryst. Growth Des., 2010, 10, 2960–2968 CrossRef CAS; (b) Q.-Y. Liu, W.-F. Wang, Y.-L. Wang, Z.-M. Shan, M.-S. Wang and J. Tang, Inorg. Chem., 2012, 51, 2381–2392 CrossRef CAS PubMed; (c) Y.-L. Wang, Y.-Y. Gao, Y. Ma, Q.-L. Wang, L.-C. Li and D.-Z. Liao, J. Solid State Chem., 2013, 202, 276–281 CrossRef CAS.
- (a) J. J. Borras-Almenar, J. M. Clemente-Juan, E. Coronado and B. S. Tsukerblat, Inorg. Chem., 1999, 38, 6081–6088 CrossRef CAS PubMed; (b) J. J. Borras-Almenar, J. M. Clemente-Juan, E. Coronado and B. S. Tsukerblat, J. Comput. Chem., 2001, 22, 985–991 CrossRef CAS.
- (a) J. P. Costes, J. M. Clemente-Juan, F. Dahan, F. Nicodeme and M. Verelst, Angew. Chem., Int. Ed., 2002, 41, 323–325 CrossRef CAS; (b) S. T. Hatscher and W. Urland, Angew. Chem., Int. Ed., 2003, 42, 2862–2864 CrossRef CAS PubMed; (c) M. Hernandez-Molina, C. Ruiz-Perez, T. Lopez, F. Lloret and M. Julve, Inorg. Chem., 2003, 42, 5456–5458 CrossRef CAS PubMed; (d) R. Baggio, R. Calvo, M. T. Garland, O. Pena, M. Perec and A. Rizzi, Inorg. Chem., 2005, 44, 8979–8987 CrossRef CAS PubMed; (e) L. Canadillas-Delgado, O. Fabelo, J. Cano, J. Pasan, F. S. Delgado, F. Lloret, M. Julve and C. Ruiz-Perez, CrystEngComm, 2009, 11, 2131–2142 RSC; (f) L. Canadillas-Delgado, O. Fabelo, J. Pasan, F. S. Delgado, F. Lloret, M. Julve and C. Ruiz-Perez, Dalton Trans., 2010, 39, 7286–7293 RSC.
- (a) J. P. Costes, J. M. C. Juan, F. Dahan and F. Nicodeme, Dalton Trans., 2003, 1272–1275 RSC; (b) L. Canadillas-Delgado, T. Martin, O. Fabelo, J. Pasan, F. S. Delgado, F. Lloret, M. Julve and C. Ruiz-Perez, Chem. – Eur. J., 2010, 16, 4037–4047 CrossRef CAS PubMed; (c) S. C. Manna, E. Zangrando, A. Bencini, C. Benelli and N. R. Chaudhuri, Inorg. Chem., 2006, 45, 9114–9122 CrossRef CAS PubMed.
- (a) F. Avecilla, C. Platas-Iglesias, R. Rodriguez-Cortinas, G. Guillemot, J. C. G. Bunzli, C. D. Brondino, C. Geraldes, A. de Blas and T. Rodriguez-Blas, Dalton Trans., 2002, 4658–4665 RSC; (b) D. Aguila, L. A. Barrios, F. Luis, A. Repolles, O. Roubeau, S. J. Teat and G. Aromi, Inorg. Chem., 2010, 49, 6784–6786 CrossRef CAS PubMed; (c) F. Pointillart, T. Cauchy, O. Maury, Y. Le Gal, S. Golhen, O. Cador and L. Ouahab, Chem. – Eur. J., 2010, 16, 11926–11941 CrossRef CAS PubMed.
- (a) K. Katoh, H. Isshiki, T. Komeda and M. Yamashita, Coord. Chem. Rev., 2011, 255, 2124–2148 CrossRef CAS; (b) K. Katoh, Y. Horii, N. Yasuda, W. Wernsdorfer, K. Toriumi, B. K. Breedlove and M. Yamashita, Dalton Trans., 2012, 41, 13582–13600 RSC; (c) Y. Wang, X.-L. Li, T.-W. Wang, Y. Song and X.-Z. You, Inorg. Chem., 2010, 49, 969–976 CrossRef CAS PubMed.
- F. Habib and M. Murugesu, Chem. Soc. Rev., 2013, 42, 3278–3288 RSC.
- (a) J. D. Rinehart1, M. Fang, W. J. Evans and J. R. Long, Nat. Chem., 2011, 3, 538–542 CrossRef PubMed; (b) J. D. Rinehart, M. Fang, W. J. Evans and J. R. Long, J. Am. Chem. Soc., 2011, 133, 14236–14239 CrossRef CAS PubMed.
- P.-F. Shi, Y.-Z. Zheng, X.-Q. Zhao, G. Xiong, B. Zhao, F.-F. Wan and P. Cheng, Chem. – Eur. J., 2012, 18, 15086–15091 CrossRef CAS PubMed.
- (a) J. J. Le Roy, M. Jeletic, S. I. Gorelsky, I. Korobkov, L. Ungur, L. F. Chibotaru and M. Murugesu, J. Am. Chem. Soc., 2013, 135, 3502–3510 CrossRef CAS PubMed; (b) M. Ren, D. Pinkowicz, M. Yoon, K. Kim, L.-M. Zheng, B. K. Breedlove and M. Yamashita, Inorg. Chem., 2013, 52, 8342–8348 CrossRef CAS PubMed; (c) F. Habib, J. Long, P.-H. Lin, I. Korobkov, L. Ungur, W. Wernsdorfer, L. F. Chibotaru and M. Murugesu, Chem. Sci., 2012, 3, 2158–2164 RSC; (d) S. K. Langley, N. F. Chilton, L. Ungur, B. Moubaraki, L. F. Chibotaru and K. S. Murray, Inorg. Chem., 2012, 51, 11873–11881 CrossRef CAS PubMed.
- (a) K. R. Meihaus, J. D. Rinehart and J. R. Long, Inorg. Chem., 2011, 50, 8484–8489 CrossRef CAS PubMed; (b) L. Zhang, P. Zhang, L. Zhao, S. Y. Lin, S. Xue, J. Tang and Z. Liu, Eur. J. Inorg. Chem., 2013, 1351–1357 CrossRef CAS.
- (a) N. Ishikawa, M. Sugita, T. Ishikawa, S. Y. Koshihara and Y. Kaizu, J. Am. Chem. Soc., 2003, 125, 8694–8695 CrossRef CAS PubMed; (b) G. J. Chen, Y. N. Guo, J. L. Tian, J. Tang, W. Gu, X. Liu, S. P. Yan, P. Cheng and D. Z. Liao, Chem. – Eur. J., 2012, 18, 2484–2487 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Selected bond lengths and angles, PXRD, TGA, VT-PXRD, IR and other magnetic measurements. CCDC 948361–948364. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4qi00116h |
| This journal is © the Partner Organisations 2014 |