Design and synthesis of nucleobase-incorporated metal–organic materials†‡
Muwei
Zhang
a,
Weigang
Lu
a,
Jian-Rong
Li
*ab,
Mathieu
Bosch
a,
Ying-Pin
Chen
ac,
Tian-Fu
Liu
a,
Yangyang
Liu
a and
Hong-Cai
Zhou
*ac
aDepartment of Chemistry, Texas A&M University, College Station, Texas 77842, USA. E-mail: zhou@mail.chem.tamu.edu; Fax: +1 979 845 1595; Tel: +1 979 845 4034
bCollege of Environmental and Energy Engineering, Beijing University of Technology, Beijing 100124, China. E-mail: jrli@bjut.edu.cn
cDepartment of Materials Science and Engineering, Texas A&M University, College Station, Texas 77842, USA
First published on 13th January 2014
Abstract
Two nucleobase-incorporated metal–organic materials were designed, synthesized and structurally characterized. PCN-530 is among the few examples of metal–organic frameworks that utilize adenine as a constructional unit, while TMOP-1 is the first existing example of a crystallographically characterized nucleobase-incorporated metal–organic polyhedron. This work also offers a general perspective for the design and synthesis of nucleobase-incorporated metal–organic materials.
Metal–organic materials (MOMs)1,2 are discrete or polymeric chemical architectures that consist of both metal units and organic linkers. While the presence of MOMs can be traced back to the 1950s,3 they have gained an increasing amount of attention in recent years. Metal–organic frameworks (MOFs)4,5 and metal–organic polyhedra (MOPs)6,7 are two important types of MOMs. Even though much effort has been put into the exploration of their novel structures and diverse applications in the past two decades, the research area of nucleobase-incorporated MOMs still remains largely under-developed. However, it is highly desirable to construct nucleobase-incorporated MOMs for the following reasons. First, materials with nucleobase moieties on its surfaces are promising materials for biological sensors8 and gene regulators.9 The traditional way of making nucleobase-incorporated materials typically involves coating DNA/RNA moieties onto the surface of gold nanoparticles.8–12 However, the majority of these materials cannot be obtained in their crystalline form. On incorporating the DNA/RNA moieties into MOMs, crystalline materials may be obtained,13–17 which should largely simplify their structural characterization and enable us to study their biological interactions. Second, the nucleobases are naturally excellent ligands. They possess multiple metal-binding sites and coordinate to metal units in various ways.18 The introduction of nucleobases into the framework can significantly enrich the coordination chemistry in MOMs and bring about intriguing structures. Third, many potential MOM applications require them to be biologically- and environmentally-friendly.19 The utilization of nucleobases will largely enhance their biological and environmental compatibility. Fourth, the production cost is always the primary concern of any industrial application of MOMs with large quantity.20 Due to their natural abundance and easy production process, the introduction of small biomolecules into MOMs may significantly bring down the production cost.
Despite these advantages associated with nucleobase-incorporated MOMs, only a limited number of nucleobase-incorporated MOFs13–17 have been published to date, and no MOPs with nucleobase moieties have been reported yet. This limitation may have resulted from the lack of intrinsic symmetry of nucleobases and the difficulty of controlling their binding modes to metal ions. Highly symmetric units are typically more favored in MOF construction21,22 since they will significantly facilitate the packing of the repetitive units in the formation of crystalline materials. The incorporation of low-symmetry units, such as nucleobases, into MOFs is usually less favorable. Rosi and co-workers have published a few “bio-MOFs” constructed from highly symmetric zinc-adeninate secondary building units (SBUs).13,14 The presence of this SBU has increased the framework symmetry and eliminated undesired coordination modes between adenine and metal ions. Apart from their approach, herein we introduce two different strategies to synthesize nucleobase-incorporated MOMs. First, despite the low symmetry of nucleobase molecules, the introduction of a highly symmetric co-ligand may be an effective way to incorporate nucleobases into MOFs. Second, nucleobase-incorporated MOPs may be constructed by connecting the nucleobase molecules to a commonly seen moiety for MOP construction. The successful implementation of these two strategies has yielded two MOMs, PCN-530 (PCN represents porous coordination network) and TMOP-1 (TMOP represents thymine-incorporated metal–organic polyhedron). Both of them are novel MOM structures with the nucleobase moieties.
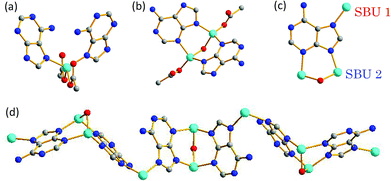
Colorless, blocky single crystals of PCN-530, Zn3[Zn2(μ2-H2O)]3(Ad)6(TATB)4(DMF), (Ad = adeninate, TATB = 4,4′,4′′-s-triazine-2,4,6-triyl-tribenzoate) were obtained via a solvothermal reaction between zinc acetate, adenine and H3TATB in N,N′-dimethylformamide (DMF) in the presence of water (see ESI‡). A single-crystal X-ray diffraction (XRD) study reveals that PCN-530 crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . Due to multiple metal-binding modes of adenine, two distinctive SBUs, denoted as “SBU 1” and “SBU 2” hereinafter, were identified in the structure (Fig. 1(a) and (b)). SBU 1 consists of a four-coordinate Zn(II) that links two adeninates via the 7-N atom on the imidazolate moiety and two carboxylates. SBU 2 consists of a Zn2(μ2-H2O) unit, where two adeninates bridge the dizinc center via 3-N and 9-N atoms at the two equatorial positions, while two carboxylates coordinate to the dizinc center at the two axial positions. It should be noted that in each adeninate, all the imino 7-N donors coordinate to SBU 1, and all the 3-N and 9-N atoms coordinate to SBU 2, leaving 1-N and the exocyclic 6-N atoms uncoordinated (Fig. 1(c)). SBU 1 and SBU 2 were connected by adeninate in a 1
. Due to multiple metal-binding modes of adenine, two distinctive SBUs, denoted as “SBU 1” and “SBU 2” hereinafter, were identified in the structure (Fig. 1(a) and (b)). SBU 1 consists of a four-coordinate Zn(II) that links two adeninates via the 7-N atom on the imidazolate moiety and two carboxylates. SBU 2 consists of a Zn2(μ2-H2O) unit, where two adeninates bridge the dizinc center via 3-N and 9-N atoms at the two equatorial positions, while two carboxylates coordinate to the dizinc center at the two axial positions. It should be noted that in each adeninate, all the imino 7-N donors coordinate to SBU 1, and all the 3-N and 9-N atoms coordinate to SBU 2, leaving 1-N and the exocyclic 6-N atoms uncoordinated (Fig. 1(c)). SBU 1 and SBU 2 were connected by adeninate in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, forming one-dimensional zinc-adeninate chains (Fig. 1(d)).
1 ratio, forming one-dimensional zinc-adeninate chains (Fig. 1(d)).
Similar to many other MOFs constructed from the TATB ligand, the s-triazine ring located in the center allows it to adopt a nearly planar conformation (Fig. 2(a)).23–25 This conformation greatly facilitates the delocalization of the π electrons within a TATB ligand and strengthens the π⋯π interaction between two adjacent ones.24 In an s-triazine core, the N and C atoms are partially negatively and positively charged, respectively. The adjacent TATB ligands stagger themselves so that the N atoms in one ligand are aligned with the C atoms of the other to maximize the π⋯π interaction.25,26 The distance between adjacent s-triazine rings is 3.53 Å (Fig. 2(a)). The one-dimensional zinc-adeninate chain is connected by TATB ligands, generating a 3,4,4-connected framework denoted as (62·84)3(63)2. The solvent accessible volume of PCN-530 is 47.80% calculated by using the PLATON routine,27 indicating its porous nature. Indeed, PCN-530 possesses open channels of 7.4 × 11.9 Å along the a axis (Fig. 2(b)).
 | ||
| Fig. 2 (a) The graphical representation of the π⋯π stacking between two TATB ligands. The dashed lines indicate the interaction between two adjacent s-triazine rings. (b) Packing diagram of PCN-530 along the a axis. High-resolution figures of the crystal structure can be found in ESI.‡ | ||
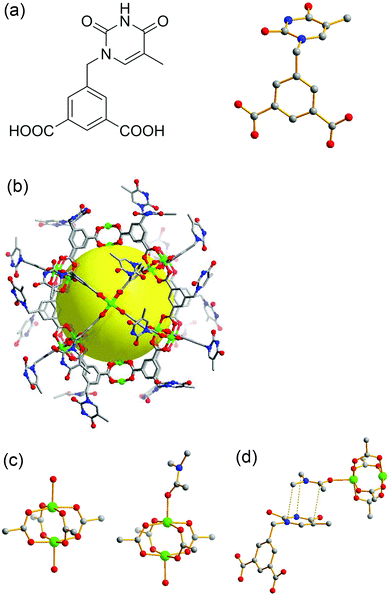
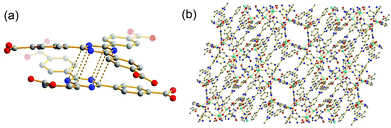
Teal, blocky crystals of TMOP-1, Cu24(MDPI)24(DMA)4(H2O)20 (MDPI = 5-((5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)methyl)isophthalate, see Fig. 3(a)), were obtained by a direct reaction between copper acetate and H2MDPI at room temperature (see ESI‡). Isophthalates are one of the important categories of MOP constructional units,28–30 and many highly porous MOFs based on cuboctahedral cavities were also constructed from ligands with isophthalate moieties.31,32 A single-crystal XRD study shows that TMOP-1 crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . Unlike the idealized Oh symmetry encountered in many other isophthalate-based MOPs,28 due to various orientations and disorders of the terminal thymine moieties, TMOP-1 possesses Ci symmetry with an inversion center located at the geometric center (Fig. 3(b)). Two types of dicopper paddlewheel SBUs were found in this structure (Fig. 3(c)), which resulted from different coordinating solvents (DMA and H2O) at the axial positions of the dicopper paddlewheels on the exterior surface of the cage. It is probable that a coordinated DMA molecule may have facilitated the packing of TMOP-1 by forming a π⋯π interaction pair with a thymine moiety from an adjacent MOP (Fig. 3(d)). The distance between the coordinated DMA and the neighboring thymine is 3.65 Å. In addition, the hydrogen bonding between two adjacent thymine moieties from two different MOPs may have facilitated the packing of TMOP-1 as well (see ESI Fig. S8‡). It should be noted that DNA-coated molecular cages were reported by Fujita and co-workers, providing neither their single crystal structures nor evidence of their crystallinity, presumably due to the complexity of their system.33 However, simple MOPs are usually regarded as crystalline materials with well-defined structures.28–30 To the best of our knowledge, this is the first reported case of nucleobase-incorporated MOP that has been structurally characterized by single crystal XRD studies.
. Unlike the idealized Oh symmetry encountered in many other isophthalate-based MOPs,28 due to various orientations and disorders of the terminal thymine moieties, TMOP-1 possesses Ci symmetry with an inversion center located at the geometric center (Fig. 3(b)). Two types of dicopper paddlewheel SBUs were found in this structure (Fig. 3(c)), which resulted from different coordinating solvents (DMA and H2O) at the axial positions of the dicopper paddlewheels on the exterior surface of the cage. It is probable that a coordinated DMA molecule may have facilitated the packing of TMOP-1 by forming a π⋯π interaction pair with a thymine moiety from an adjacent MOP (Fig. 3(d)). The distance between the coordinated DMA and the neighboring thymine is 3.65 Å. In addition, the hydrogen bonding between two adjacent thymine moieties from two different MOPs may have facilitated the packing of TMOP-1 as well (see ESI Fig. S8‡). It should be noted that DNA-coated molecular cages were reported by Fujita and co-workers, providing neither their single crystal structures nor evidence of their crystallinity, presumably due to the complexity of their system.33 However, simple MOPs are usually regarded as crystalline materials with well-defined structures.28–30 To the best of our knowledge, this is the first reported case of nucleobase-incorporated MOP that has been structurally characterized by single crystal XRD studies.
Conclusions
In conclusion, we have synthesized and characterized two novel nucleobase-incorporated MOMs, PCN-530 and TMOP-1. PCN-530 was prepared by introducing a highly symmetric linker as the co-ligand during the formation of MOFs, to compensate for the low-symmetry nature of adenine. It possesses two distinctive rarely seen SBUs. TMOP-1 was prepared by incorporating thymine into the isophthalate moiety, which is commonly used for MOP syntheses. It is the first crystallographically confirmed example of nucleobase-incorporated MOP. It should be noted that in both PCN-530 and TMOP-1, the nucleobase hydrogen bonding sites are open, which makes it possible to incorporate DNA base pair interaction into the MOMs. This work has shown some potential in making DNA-coated biosensors and it is currently in progress in our lab. More importantly, the successful syntheses of PCN-530 and TMOP-1 also provide general guidance for the future design of nucleobase-incorporated MOMs.Acknowledgements
This work was supported as part of the Center for Gas Separations Relevant to Clean Energy Technologies, an Energy Frontier Research Center funded by the U.S. Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences under Award Number DE-SC0001015. The authors also acknowledge the DOE grant DE-FC36-07GO17033 and the Welch Foundation A-1725.Notes and references
- J. J. Perry IV, J. A. Perman and M. J. Zaworotko, Chem. Soc. Rev., 2009, 38, 1400–1417 RSC.
- A. Carne, C. Carbonell, I. Imaz and D. Maspoch, Chem. Soc. Rev., 2011, 40, 291–305 RSC.
- J. H. Rayner and H. M. Powell, J. Chem. Soc., 1952, 319–328 RSC.
- H.-C. Zhou, J. R. Long and O. M. Yaghi, Chem. Rev., 2012, 112, 673–674 CrossRef CAS PubMed.
- M. Zhang, Y.-P. Chen, M. Bosch, T. Gentle, K. Wang, D. Feng, Z. U. Wang and H.-C. Zhou, Angew. Chem., Int. Ed., 2013 DOI:10.1002/anie.201307340 , ASAP.
- J.-R. Li, D. J. Timmons and H.-C. Zhou, J. Am. Chem. Soc., 2009, 131, 6368–6369 CrossRef CAS PubMed.
- M. J. Zaworotko, Nat. Chem., 2009, 1, 267–268 CrossRef CAS PubMed.
- R. Elghanian, J. J. Storhoff, R. C. Mucic, R. L. Letsinger and C. A. Mirkin, Science, 1997, 277, 1078–1081 CrossRef CAS.
- D. A. Giljohann, D. S. Seferos, A. E. Prigodich, P. C. Patel and C. A. Mirkin, J. Am. Chem. Soc., 2009, 131, 2072–2073 CrossRef CAS PubMed.
- N. L. Rosi, D. A. Giljohann, C. S. Thaxton, A. K. R. Lytton-Jean, M. S. Han and C. A. Mirkin, Science, 2006, 312, 1027–1030 CrossRef CAS PubMed.
- T. A. Taton, C. A. Mirkin and R. L. Letsinger, Science, 2000, 289, 1757–1760 CrossRef CAS.
- Y. Shan, J.-J. Xu and H.-Y. Chen, Chem. Commun., 2009, 905–907 RSC.
- J. An, O. K. Farha, J. T. Hupp, E. Pohl, J. I. Yeh and N. L. Rosi, Nat. Commun., 2012, 3, 604 CrossRef PubMed.
- J. An, S. J. Geib and N. L. Rosi, J. Am. Chem. Soc., 2009, 131, 8376–8377 CrossRef CAS PubMed.
- J. An, S. J. Geib and N. L. Rosi, J. Am. Chem. Soc., 2009, 132, 38–39 CrossRef PubMed.
- T. Li, M. T. Kozlowski, E. A. Doud, M. N. Blakely and N. L. Rosi, J. Am. Chem. Soc., 2013, 135, 11688–11691 CrossRef CAS PubMed.
- P. S. Nugent, V. L. Rhodus, T. Pham, K. Forrest, L. Wojtas, B. Space and M. J. Zaworotko, J. Am. Chem. Soc., 2013, 135, 10950–10953 CrossRef CAS PubMed.
- D. K. Patel, A. Domínguez-Martín, M. d. P. Brandi-Blanco, D. Choquesillo-Lazarte, V. M. Nurchi and J. Niclós-Gutiérrez, Coord. Chem. Rev., 2012, 256, 193–211 CrossRef CAS PubMed.
- I. Imaz, M. Rubio-Martinez, J. An, I. Sole-Font, N. L. Rosi and D. Maspoch, Chem. Commun., 2011, 47, 7287–7302 RSC.
- M. Zhang, Y.-P. Chen and H.-C. Zhou, CrystEngComm, 2013, 15, 9544–9552 RSC.
- D. Zhao, D. J. Timmons, D. Yuan and H.-C. Zhou, Acc. Chem. Res., 2010, 44, 123–133 CrossRef PubMed.
- F. A. Almeida Paz, J. Klinowski, S. M. F. Vilela, J. P. C. Tome, J. A. S. Cavaleiro and J. Rocha, Chem. Soc. Rev., 2012, 41, 1088–1110 RSC.
- S. Ma and H.-C. Zhou, J. Am. Chem. Soc., 2006, 128, 11734–11735 CrossRef CAS PubMed.
- D. Sun, S. Ma, Y. Ke, D. J. Collins and H.-C. Zhou, J. Am. Chem. Soc., 2006, 128, 3896–3897 CrossRef CAS PubMed.
- D. Sun, S. Ma, Y. Ke, T. M. Petersen and H.-C. Zhou, Chem. Commun., 2005, 2663–2665 RSC.
- V. R. Thalladi, R. Boese, S. Brasselet, I. Ledoux, J. Zyss, R. K. R. Jetti and G. R. Desiraju, Chem. Commun., 1999, 1639–1640 RSC.
- A. Spek, J. Appl. Crystallogr., 2003, 36, 7–13 CrossRef CAS.
- M. Eddaoudi, J. Kim, J. B. Wachter, H. K. Chae, M. O'Keeffe and O. M. Yaghi, J. Am. Chem. Soc., 2001, 123, 4368–4369 CrossRef CAS.
- J.-R. Li, A. A. Yakovenko, W. Lu, D. J. Timmons, W. Zhuang, D. Yuan and H.-C. Zhou, J. Am. Chem. Soc., 2010, 132, 17599–17610 CrossRef CAS PubMed.
- J.-R. Li and H.-C. Zhou, Nat. Chem., 2010, 2, 893–898 CrossRef CAS PubMed.
- D. Yuan, D. Zhao, D. Sun and H.-C. Zhou, Angew. Chem., Int. Ed., 2010, 49, 5357–5361 CrossRef CAS PubMed.
- O. K. Farha, I. Eryazici, N. C. Jeong, B. G. Hauser, C. E. Wilmer, A. A. Sarjeant, R. Q. Snurr, S. T. Nguyen, A. Ö. Yazaydın and J. T. Hupp, J. Am. Chem. Soc., 2012, 134, 15016–15021 CrossRef CAS PubMed.
- T. Kikuchi, S. Sato and M. Fujita, J. Am. Chem. Soc., 2010, 132, 15930–15932 CrossRef CAS PubMed.
Footnotes |
| † This work is dedicated to Prof. Dr Xiao-Zeng You on the occasion of his 80th birthday. |
| ‡ Electronic supplementary information (ESI) available: Detailed synthetic procedures of the ligands, the preparation of PCN-530 and TMOP-1, their structure solution and refinement, and high-resolution figures for their crystal structures can be found in the ESI. CCDC 962335 for PCN-530, 962336 for TMOP-1. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3qi00042g |
| This journal is © the Partner Organisations 2014 |