Self-assembled nanoparticles from thiol functionalized liquid crystalline brush block copolymers for dual encapsulation of doxorubicin and gold nanoparticles†
Abstract
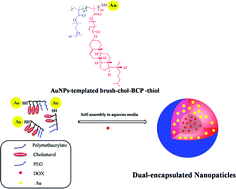
We synthesized new amphiphilic brush liquid crystalline block copolymers (brush-chol-BCP) comprised of polymethacrylates bearing polyethylene oxide (PEO) in one block and polymethacrylates bearing a cholesterol mesogen with a hemitelechelic thiol end group. Polymethacrylate bearing PEO (PMA-g-PEO) was first synthesized by reversible addition-fragmentation chain transfer polymerization (RAFT) and used as a macro-chain transfer agent to prepare block copolymer (PMA-g-PEO)-b-PC5MA-thioester (brush-chol-BCP-thioester). Brush-chol-BCP-thiol was obtained by the reduction of a thioester to thiol in the presence of butylamine. Gold nanoparticles (AuNPs) were prepared in situ with the brush-chol-BCP-thiol template via the reduction of gold ions and were stabilized by directly anchoring to the brush-chol-BCP-thiol chains through the coordination bonds with the thiol groups in the copolymer. The hydrophobic anticancer drug doxorubicin (DOX) was successfully encapsulated into AuNP-templated brush-chol-BCP-thiol via physical entrapment to form dual-encapsulated NPs with a high drug loading of 21.4% (w/w) and a high encapsulation efficiency of 85.6%. The dual-encapsulated NPs had an average size of 157 nm, spherical shape, excellent stability, and a sustained drug release pattern. More importantly, the dual-encapsulated NPs could be effectively internalized by human cervical cancer cells (Hela) and showed dose-dependent cytotoxicity, while the blank nanoparticles were non-cytotoxic at the tested concentrations. The results indicate that the brush-chol-BCP-thiol and their nanoparticles are promising carriers for dual encapsulation and delivery of an anticancer drug and metal nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...