Porphyrin-containing hyperbranched supramolecular polymers: enhancing 1O2-generation efficiency by supramolecular polymerization†
Yiliu
Liu‡
a,
Zehuan
Huang‡
a,
Kai
Liu
a,
Hans
Kelgtermans
b,
Wim
Dehaen
b,
Zhiqiang
Wang
*a and
Xi
Zhang
*a
aThe Key Lab of Organic Optoelectronics & Molecular Engineering, Department of Chemistry, Tsinghua University, Beijing 100084, P. R. China. E-mail: wangzhiqiang@mail.tsinghua.edu.cn; xi@mail.tsinghua.edu.cn
bMolecular Design and Synthesis, Department of Chemistry KU Leuven, Leuven, Belgium
First published on 22nd August 2013
Abstract
Hyperbranched supramolecular polymers were obtained by mixing a naphthyl-substituted porphyrin derivative and cucurbit[8]uril in aqueous solution, which was driven by host–guest interactions. The formation of a supramolecular polymeric structure can cause disruption of the porphyrin aggregation, thus leading to enhancement of their 1O2-generation efficiency.
Supramolecular polymers are polymers whose monomers are connected through directional and reversible noncovalent interactions.1–3 By virtue of their polymeric structure and noncovalent nature, supramolecular polymers hold great potential for use as dynamic functional materials.4–6 In the past two decades, a rapid evolution of supramolecular polymer chemistry has been achieved, which mainly involves two streams of efforts. One is to develop driving forces for supramolecular polymerization, such as hydrogen bonding, metal-coordination, and host–guest interactions.7–16 The other is to approach practical applications of supramolecular polymers, for example, using supramolecular polymers as degradable and self-healing materials.17–21
Recently, we have employed cucurbit[8]uril (CB[8])-based host–guest interactions22–24 as driving forces to fabricate a series of water-soluble supramolecular polymers.25–27 The high binding constants of CB[8]-based host–guest interactions facilitate supramolecular polymerization under low monomer concentration. For example, CB[8] can encapsulate two naphthalene moieties inside its cavity to form a ternary complex, the ternary equilibrium constants can reach 1011 to 1012 M−2.28 It has been successfully employed as a driving force to construct linear and hyperbranched supramolecular polymers.29–31 On the basis of these findings, we attempt to take this a step further to incorporate functional building blocks into the systems to fabricate functional supramolecular polymers. In this communication, we report employing CB[8]-naphthalene host–guest interactions to construct a type of porphyrin-containing hyperbranched supramolecular polymers which can function as photosensitizers. We further demonstrate that the formation of supramolecular polymers can cause disruption of porphyrin aggregation, which leads to a significant enhancement of the 1O2-generation efficiency.
To fabricate hyperbranched supramolecular polymers, monomer (TPOR) which contains four naphthyl-substituted arms and a porphyrin core was designed and synthesized (Scheme 1). The four naphthyl-substituted arms are designed to bind with CB[8] to form hyperbranched supramolecular polymers. In addition, the porphyrin core is introduced as a functional unit to make the hyperbranched supramolecular polymers as photosensitizers.32–35 A problem here to be addressed is that porphyrins have a strong tendency to aggregate in aqueous solution, which leads to a significant decrease in their activity.36,37 To solve this problem, a short and rigid methylene spacer between the naphthalene moiety and the porphyrin core is designed. In this case, when the bulky CB[8] molecules bind with the naphthalene moieties, the close stacking of porphyrins can be impeded due to steric effects.
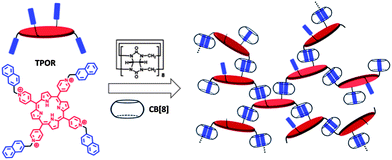 | ||
| Scheme 1 Schematic diagram of the formation of hyperbranched supramolecular polymers through self-assembly of TPOR and CB[8]. | ||
We wondered if the host–guest interaction between TPOR and CB[8] was strong enough to drive the supramolecular polymerization. Isothermal titration calorimetry (ITC) was used to answer this question. A solution of TPOR was consecutively injected into a solution of CB[8] and an exothermic binding isotherm was recorded (Fig. 1). The titration curve displayed a drastic change when TPOR and CB[8] were mixed in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. This binding stoichiometry is consistent with the supramolecular polymerization protocol. Fitting the calorimetric data by a two-site binding model gave a binding constant of 1.6 × 1012 M−2 (Fig. S1†), which indicates that TPOR and CB[8] can indeed strongly bind together through host–guest interactions.
2. This binding stoichiometry is consistent with the supramolecular polymerization protocol. Fitting the calorimetric data by a two-site binding model gave a binding constant of 1.6 × 1012 M−2 (Fig. S1†), which indicates that TPOR and CB[8] can indeed strongly bind together through host–guest interactions.
1H NMR spectroscopy was employed to give further information about the formation of supramolecular polymers. As indicated in Fig. 2, after addition of CB[8] into TPOR solution, all naphthyl protons on TPOR exhibited a significant upfield shift and no end-group signals could be distinguished, indicating that the naphthalene moieties are efficiently encapsulated by CB[8] to form hyperbranched supramolecular polymers. Diffusion-ordered NMR spectroscopy (DOSY), which can provide samples' diffusion coefficient (D), was further used to confirm the formation of hyperbranched supramolecular polymers.38 The average diffusion coefficients (Davg) of TPOR and CB[8] were found to be 2.27 × 10−10 m2 s−1 and 2.93 × 10−10 m2 s−1, respectively. However, in the TPOR–2CB[8] solution, the NMR signals of both TPOR and CB[8] show a single diffusion coefficient with a smaller value of Davg = 8.40 × 10−11 m2 s−1. This significant decrease in diffusion coefficient implies that large-size hyperbranched supramolecular polymers have formed indeed.
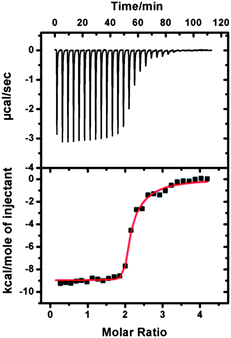 | ||
| Fig. 1 ITC data for the titration of CB[8] (0.05 mM) with TPOR (0.25 mM) in buffered aqueous solution at 25 °C. | ||
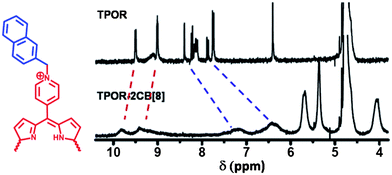 | ||
| Fig. 2 1H NMR spectra of TPOR and TPOR–2CB[8] hyperbranched supramolecular polymers in D2O at 25 °C. | ||
The ability of TPOR–2CB[8] hyperbranched supramolecular polymers to act as photosensitizers was then investigated by evaluating their 1O2-generation efficiency. Electron paramagnetic resonance (EPR) spectroscopy was employed to monitor the generation of 1O2. 2,2,6,6-Tetramethylpiperidine (TEMP), which can capture 1O2 to yield paramagnetic nitroxide radical TEMPO, was chosen as the EPR probe (Fig. 3).39 TPOR–2CB[8] and TPOR show equal signals without photoirradiation (Fig. S2†). However, upon photoirradiation for 30 min, the TPOR–2CB[8] hyperbranched supramolecular polymers showed a much stronger EPR signal than TPOR by itself (Fig. 3a). A clearer contrast of kinetics was shown with an EPR intensity–time plot (Fig. 3b). By comparing the slope of the curves, the 1O2 generation rate for the TPOR–2CB[8] hyperbranched supramolecular polymers is much faster compared with that of TPOR. Since CB[8] alone cannot generate 1O2 upon irradiation, the enhanced efficiency of the TPOR–2CB[8] to generate 1O2 should be attributed to the formation of hyperbranched supramolecular polymers.
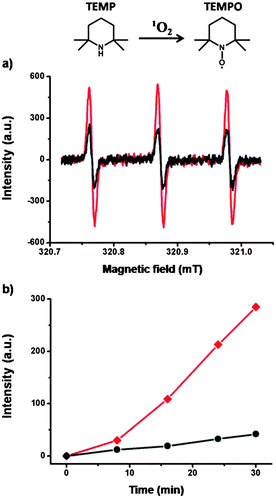 | ||
| Fig. 3 (a) EPR spectra upon irradiation of 30 min and (b) the change of intensity of the EPR peak with irradiation time of TPOR–2CB[8] hyperbranched supramolecular polymers (red) and TPOR (black). | ||
One plausible explanation is that in the TPOR–2CB[8] hyperbranched supramolecular polymers, the bulky and space-demanding CB[8] molecules can bind with the naphthalene moieties that are adjacent to porphyrins, thus impeding the close stacking of the porphyrins by steric effects. UV-Vis spectroscopy and fluorescence spectroscopy were employed to verify this assumption (Fig. 4). Addition of CB[8] into an aqueous solution of TPOR caused a decrease of absorption around 220 nm, which can be ascribed to encapsulation of naphthalene moieties inside the CB[8] cavity. Meanwhile, the TPOR absorption around 425 nm, which can be assigned as the Soret band of the porphyrin moieties, shows an increase. Considering the molar extinction coefficient of the chromophores is dependent on the distance of the adjacent chromophores, the observed increase of porphyrin absorption indicates that the distance between porphyrin moieties is increased. The results of fluorescence spectroscopy also support the assumption. After adding CB[8], the fluorescence of TPOR enhances significantly (Fig. S3†). It indicates that the close stacking of the porphyrin moieties is disrupted, thus leading to the enhancement of the fluorescence.
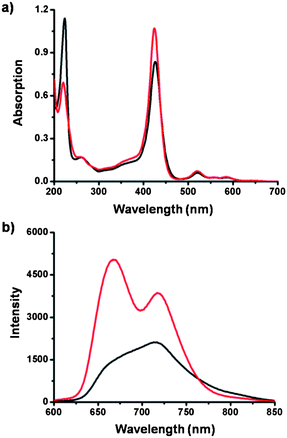 | ||
| Fig. 4 (a) UV-Vis spectra and (b) fluorescence spectra of TPOR–2CB[8] (5.0 μM, red) hyperbranched supramolecular polymers and TPOR (5.0 μM, black). | ||
By virtue of the noncovalent nature, the TPOR–2CB[8] hyperbranched supramolecular polymers can be readily depolymerised as well. 1-Adamantanamine hydrochloride (AD), which can compete with TPOR for CB[8], was used as a depolymerisation agent. After adding two equivalents of AD into the solution of TPOR–2CB[8], the average diffusion coefficient was increased to 2.16 × 10−10 m2 s−1 and the fluorescence of the solution recovered to the quenched state, indicating that the supramolecular hyperbranched polymers are depolymerised. Owing to such a dynamic feature, TPOR–2CB[8] hyperbranched supramolecular polymers may be used as reversible and adaptive photosensitizers that can be activated and deactivated on demand.
Conclusions
In summary, for the first time we have successfully fabricated a type of porphyrin-containing hyperbranched supramolecular polymers that can function as photosensitizers. The formation of the supramolecular polymeric structure can disrupt the aggregation of the porphyrins, thus improving the 1O2 generation efficiency. It is anticipated that such supramolecular polymer based photosensitizers may find their further application, for example, in wastewater treatment, fine chemical synthesis and photodynamic therapy.40Acknowledgements
This work was financially supported by the NSFC (21274076), NSFC-DFG joint grant (TRR61), the NSFC Innovative Group (21121004), National Basic Research Program of China (2013CB834502) and Bilateral Scientific Cooperation between Tsinghua University & K. U. Leuven.Notes and references
- C. Fouquey, J.-M. Lehn and A. M. Levelut, Adv. Mater., 1990, 2, 254 CrossRef CAS.
- R. P. Sijbesma, F. L. Beijer, L. Brunsveld, B. J. B. Folmer, J. H. K. K. Hirschberg, R. F. M. Lange, J. K. L. Lowe and E. W. Meijer, Science, 1997, 278, 1601 CrossRef CAS.
- L. Brunsveld, B. J. B. Folmer, E. W. Meijer and R. P. Sijbesma, Chem. Rev., 2001, 101, 4071 CrossRef CAS PubMed.
- T. F. A. de Greef and E. W. Meijer, Nature, 2008, 453, 171 CrossRef CAS PubMed.
- T. Aida, E. W. Meijer and S. I. Stupp, Science, 2012, 335, 813 CrossRef CAS PubMed.
- X. Yan, F. Wang, B. Zheng and F. Huang, Chem. Soc. Rev., 2012, 41, 6042 RSC.
- L. Bouteiller, Adv. Polym. Sci., 2007, 207, 79 CrossRef CAS.
- C. Schmuck and W. Wienand, Angew. Chem., Int. Ed., 2001, 40, 4363 CrossRef CAS.
- R. Knapp, A. Schott and M. Rehahn, Macromolecules, 1996, 29, 478 CrossRef CAS.
- M. Chiper, R. Hoogenboom and U. S. Schubert, Macromol. Rapid Commun., 2009, 30, 565 CrossRef CAS PubMed.
- B. Zheng, F. Wang, S. Dong and F. Huang, Chem. Soc. Rev., 2012, 41, 1621 RSC.
- D. Guo and Y. Liu, Chem. Soc. Rev., 2012, 41, 5907 RSC.
- A. Harada, Y. Takashima and H. Yamaguchi, Chem. Soc. Rev., 2009, 38, 875 RSC.
- R. Sun, C. Xue, X. Ma, M. Gao, H. Tian and Q. Li, J. Am. Chem. Soc., 2013, 135, 5990 CrossRef CAS PubMed.
- M. Sun, H. Zhang, B. Liu and Y. Liu, Macromolecules, 2013, 46, 4268 CrossRef CAS.
- S. Li, T. Xiao, C. Lin and L. Wang, Chem. Soc. Rev., 2012, 41, 5950 RSC.
- P. Cordier, F. Tournilhac, C. Soulie-Ziakovic and L. Leibler, Nature, 2008, 451, 977 CrossRef CAS PubMed.
- M. Burnworth, L. Tang, J. R. Kumpfer, A. J. Duncan, F. L. Beyer, G. L. Fiore, S. J. Rowan and C. Weder, Nature, 2011, 472, 334 CrossRef CAS PubMed.
- B. C. Tee, C. Wang, R. Allen and Z. Bao, Nat. Nanotechnol., 2012, 7, 825 CrossRef CAS PubMed.
- X. Yan, D. Xu, J. Chen, M. Zhang, B. Hu, Y. Yu and F. Huang, Polym. Chem., 2013, 4, 3312 RSC.
- L. R. Hart, J. L. Harries, B. W. Greenland, H. M. Colquhoun and W. Hayes, Polym. Chem., 2013, 4, 4860 RSC.
- Y. H. Ko, E. Kim, I. Hwang and K. Kim, Chem. Commun., 2007, 1305 RSC.
- J. Lagona, P. Mukhopadhyay, S. Chakrabarti and L. Isaacs, Angew. Chem., Int. Ed., 2005, 44, 4844 CrossRef CAS PubMed.
- D. Das and O. A. Scherman, Isr. J. Chem., 2011, 51, 537 CrossRef CAS.
- Y. Liu, H. Yang, Z. Wang and X. Zhang, Chem.–Asian J., 2013, 8, 1626 CrossRef CAS PubMed.
- Y. Liu, Y. Yu, J. Gao, Z. Wang and X. Zhang, Angew. Chem., Int. Ed., 2010, 49, 6576 CrossRef CAS PubMed.
- X. Tan, L. Yang, Y. Liu, Z. Huang, H. Yang, Z. Wang and X. Zhang, Polym. Chem., 2013 10.1039/C3PY00888F.
- D. Jiao, F. Biedermann, F. Tian and O. A. Scherman, J. Am. Chem. Soc., 2010, 132, 15734 CrossRef CAS PubMed.
- Y. Liu, R. Fang, X. Tan, Z. Wang and X. Zhang, Chem.–Eur. J., 2012, 18, 15650 CrossRef CAS PubMed.
- Y. Liu, Z. Huang, X. Tan, Z. Wang and X. Zhang, Chem. Commun., 2013, 49, 5766 RSC.
- R. Fang, Y. Liu, Z. Wang and X. Zhang, Polym. Chem., 2013, 4, 900 RSC.
- R. Bonnett, Chem. Soc. Rev., 1995, 24, 19 RSC.
- R. K. Pandey, K. M. Smith and T. J. Dougherty, J. Med. Chem., 1990, 33, 2032 CrossRef CAS.
- N. Nishiyama, H. R. Stapert, G. Zhang, D. Takasu, D. Jiang, T. Nagano, T. Aida and K. Kataoka, Bioconjugate Chem., 2003, 14, 58 CrossRef CAS PubMed.
- C. Xing, Q. Xu, H. Tang, L. Liu and S. Wang, J. Am. Chem. Soc., 2009, 131, 13117 CrossRef CAS PubMed.
- K. Liu, Y. Liu, Y. Yao, H. Yuan, S. Wang, Z. Wang and X. Zhang, Angew. Chem., Int. Ed., 2013, 52, 8285 CrossRef CAS PubMed.
- J. Voskuhl, U. Kauscher, M. Gruener, H. Frisch, B. Wibbeling, C. A. Strassert and B. J. Ravoo, Soft Matter, 2013, 9, 2453 RSC.
- Y. Liu, Z. Wang and X. Zhang, Chem. Soc. Rev., 2012, 41, 5922 RSC.
- J. Moan and E. Wold, Nature, 1979, 279, 450 CrossRef CAS PubMed.
- M. C. DeRosa and R. J. Crutchley, Coord. Chem. Rev., 2002, 233–234, 351 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3py01036h |
| ‡ These two authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2014 |
