Unusual regio- and stereo-selectivity in Diels–Alder reactions between bulky N-phenylmaleimides and anthracene derivatives†
Abstract
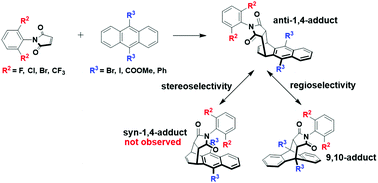
Unusual regio- and stereo-selectivity in Diels–Alder (D–A) reactions were achieved between bulky N-phenylmaleimides and anthracene derivatives. Using multiple substituents with steric hindrance on both diene and dienophile, a noticeable shift toward 1,4-addition was successfully obtained. The substrate scope in this reaction was broad and the highest yield of anti-1,4-adducts was over 90%. Novel structures of anti-1,4-adducts were confirmed by single crystal X-ray diffraction analysis. This study not only provides the first reported method of synthesizing anti-1,4-adducts and achieving otherwise unattainable regio- and stereo-selectivity, but also elucidates the importance of combining the steric effects of two reactants to shift products toward 1,4-adducts. Moreover, the resulting 1,4-adducts could be further functionalized through their halogen groups via carbon–carbon coupling reactions.

 Please wait while we load your content...
Please wait while we load your content...