The nature of persistent conformational chirality, racemization mechanisms, and predictions in diarylether heptanoid cyclophane natural products†
Abstract
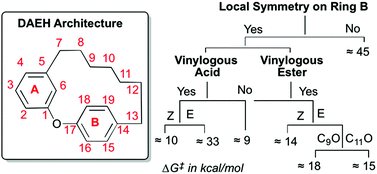
Restricted rotations of chemical bonds can lead to the presence of persistent conformational chirality in molecules lacking stereocenters. We report the development of first-of-a-kind predictive rules that enable identification of conformational chirality and prediction of racemization barriers in the diarylether heptanoid (DAEH) natural products that do not possess stereocenters. These empirical rules-of-thumb are based on quantum mechanical computations (SCS-MP2/∞//B3LYP/6-31G*/PCM) of racemization barriers of four representative DAEHs. Specifically, the local symmetry of ring B and the E/Z configuration of the vinylogous acid/ester are critical in determining conformational chirality in the DAEH natural product family.

 Please wait while we load your content...
Please wait while we load your content...