Synthesis of four-armed triphenylamine-based molecules and their applications in organic solar cells†
Abstract
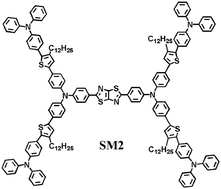
Two wide band gap four-armed triphenylamine (TPA)-based molecules (SM1 and SM2) with a donor–acceptor–donor structural core and a thiophene (Th) or TPA-Th segment as the arm have been designed and synthesized by use of Stille coupling reaction to further study the relationship between the structure and properties of four-armed triphenylamine-based molecules. The thermal, photophysical, electrochemical and photovoltaic properties of the molecules are studied. To further study the electronic structure of these molecules, time-dependent density functional theory calculations for the resulting small molecules were performed by using the Gaussian 09 program suite. SM2 with the extended arm structures has stronger absorption intensity and better miscibility with commercial PCBM compared with the SM1 molecule. The organic solar cells employing SM2/[6,6]-phenyl-C71-butyric acid methyl ester (PC71BM) as the active layer showed better device performances than that of SM1.

 Please wait while we load your content...
Please wait while we load your content...