NiII–Schiff base complex as an enzyme inhibitor of hen egg white lysozyme: a crystallographic and spectroscopic study†
Abstract
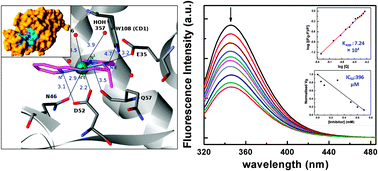
The engineering of protein–small molecule interactions becomes imperative today to recognize the essential biochemical processes in living systems. Here we have investigated the interaction between hen egg white lysozyme (HEWL) and a newly synthesized small, simple nickel Schiff base complex (NSC) {(N1E,N2E)-N1,N2-bis(pyridine-2-ylmethylene)propane-1,2-diaminenickel(II)} using different spectroscopic techniques. We attempted to determine the exact site of the interaction by crystallography. Absorption spectroscopy reveals that the interaction occurs through the ground state. The complex can quench the intrinsic fluorescence of HEWL through a static quenching method. The fluorescence quenching study along with the determination of thermodynamic parameters reveal that NSC binds HEWL spontaneously with moderate binding affinity. The results have also identified that the spontaneity of this enthalpy guided interaction is mainly governed by some H-bonding and hydrophobic interactions which are also indicated by the crystallographic analyses. Moreover, the crystallographic study shows that NSC makes its way into the active site enzyme cavity of HEWL forming a single covalent adduct between Ni2+ and the oxygen of the active site Asp 52. The possibility of inhibiting the catalytic activity of HEWL by inclusion of NSC in the enzyme active site observed from crystallographic analyses has also been confirmed by enzyme kinetics experiments.

 Please wait while we load your content...
Please wait while we load your content...