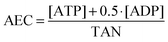Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
In vitro toxicological characterisation of three arsenic-containing hydrocarbons
S.
Meyer
ab,
M.
Matissek
c,
S. M.
Müller
c,
M. S.
Taleshi
d,
F.
Ebert
b,
K. A.
Francesconi
d and
T.
Schwerdtle
*b
aGraduate School of Chemistry, University of Münster, Wilhelm-Klemm-Straße 10, 48149 Münster, Germany
bInstitute of Nutritional Science, University of Potsdam, Arthur-Scheunert-Allee 114-116, 14558 Nuthetal, Germany. E-mail: Tanja.Schwerdtle@uni-potsdam.de
cInstitute of Food Chemistry, University of Münster, Corrensstraße 45, 48149 Münster, Germany
dInstitute of Chemistry – Analytical Chemistry, University of Graz, Universitätsplatz 1, 8010 Graz, Austria
First published on 20th March 2014
Abstract
Arsenic-containing hydrocarbons are one group of fat-soluble organic arsenic compounds (arsenolipids) found in marine fish and other seafood. A risk assessment of arsenolipids is urgently needed, but has not been possible because of the total lack of toxicological data. In this study the cellular toxicity of three arsenic-containing hydrocarbons was investigated in cultured human bladder (UROtsa) and liver (HepG2) cells. Cytotoxicity of the arsenic-containing hydrocarbons was comparable to that of arsenite, which was applied as the toxic reference arsenical. A large cellular accumulation of arsenic, as measured by ICP-MS/MS, was observed after incubation of both cell lines with the arsenolipids. Moreover, the toxic mode of action shown by the three arsenic-containing hydrocarbons seemed to differ from that observed for arsenite. Evidence suggests that the high cytotoxic potential of the lipophilic arsenicals results from a decrease in the cellular energy level. This first in vitro based risk assessment cannot exclude a risk to human health related to the presence of arsenolipids in seafood, and indicates the urgent need for further toxicity studies in experimental animals to fully assess this possible risk.
Introduction
In the environment arsenic occurs ubiquitously in many inorganic and organic species. Up to now more than 50 As species have been identified.1For the general population food is the major source of arsenic. However, the arsenic content in terrestrial food (excluding rice) is quiet low, and inorganic arsenic (iAs) is the predominant arsenic form. iAs has been classified as a human carcinogen (group 1) by the International Agency for Research on Cancer (IARC). After chronic ingestion as well as inhalative exposure, iAs causes tumours of the lung, skin and bladder.2,3 To date the underlying molecular mechanisms of iAs-induced carcinogenicity are still not fully understood.
In marine fish and other seafood the total arsenic content is in general much higher than that in terrestrial food. Furthermore, in seafood organic arsenic compounds are the major arsenicals, including the water-soluble arsenobetaine or arsenosugars as well as the lipid-soluble arsenolipids.4 In general the fat fraction of livers and other organs of marine fish is rich in arsenic. Oils from these marine fish generally contain between 1 and 50 mg As kg−1 oil. Therefore the lipid-soluble arsenic compounds are around 10–30% of the total arsenic present in marine organisms.5 In cod liver samples up to 77% of the total arsenic (3.3 mg kg−1 dry mass)6 and in tuna around 50% of the total arsenic (5.9 mg kg−1 dry mass) have been identified as lipid-extractable.4
Toxicological evaluation of arsenobetaine indicates that this arsenic compound is non-toxic to humans. In humans after oral intake, arsenobetaine is excreted unchanged via urine.7,8
In contrast to arsenobetaine, arsenolipids seem to be efficiently metabolised by humans. Thus, after ingestion of arsenolipids present in cod liver oil by two volunteers, dimethylarsinic acid (DMAV) was identified as the major metabolite (up to 70%).6,9 DMAV also represents the major metabolite of iAsIII. DMAV has been shown to exert genotoxicity in cultured mammalian cells (e.g.ref. 10–12), to induce bladder cancer in rats13 and has been classified by the IARC in 2012 as possibly carcinogenic to humans (group 2B).14 The toxicity of arsenolipids has not been investigated, and hence there are no toxicological data to assess the risks to human health related to the presence of arsenolipids in seafood.3
Although the occurrence of arsenolipids in fish and other types of seafood was first reported in the 1960s,15 little was known about the structures of these compounds for many years afterwards. The first arsenolipid was identified in 1988 as an arsenosugar bound to a phospholipid.16 Through the improvement of analytical techniques more arsenolipids have been found in the last couple of years, especially in fish and algae. Two other groups of arsenolipids could be identified in fish oils, which were categorised into arsenic-containing hydrocarbons (AsHC)17 and arsenic-containing long-chain fatty acids (AsFA).18 Recently, further structures of arsenosugar-phospholipids (AsPL) have been reported in brown algae.19
This study focuses on the cellular toxicity of three arsenic-containing hydrocarbons (AsHC 332, AsHC 360, and AsHC 444 (Fig. 1)). These arsenicals have been identified in fish oil,17,20,21 but also in several fish meat samples, including tuna,4 cod22,23 and herring,24 as well as in some brown algae.19 For the in vitro cellular system, immortalised human urothelium cells (UROtsa) were applied, since the bladder has been identified as the target tissue for iAs and DMAV induced toxicity. For the second model system, liver cells (HepG2) were used, since after intestinal absorption the arsenicals are likely to be metabolised in the liver. In the first step the cytotoxic profiles of the arsenolipids were compared to the profile of the toxic reference compound iAsIII. Since all three arsenic-containing hydrocarbons exerted strong cytotoxic effects, further studies were carried out to identify the toxic mode of action of the respective arsenolipids. Endpoints included among others cellular bioavailability and distribution of the arsenicals, genotoxicity as well as effects on the cellular energy level.
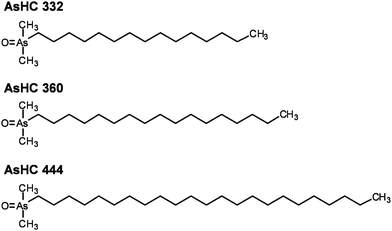 | ||
| Fig. 1 Chemical structures and abbreviations of three arsenic-containing hydrocarbons investigated in this study. | ||
Experimental
Material
Minimal essential medium Eagle (MEM), nonessential amino acids and the culture dishes were supplied by Biochrom (Berlin, Germany). Fetal calf serum (FCS), penicillin–streptomycin solutions and trypsin were products of PAA Laboratories GmbH (Pasching, Austria). Triton X-100 was purchased from Thermo Scientific (Rockford, USA), hydroxyapatite (high resolution) and potassium dihydrogen phosphate from Calbiochem (Bad Soden, Germany) and Giemsa stain, hydrogen peroxide solution (30%, suprapur), nitric acid (65% suprapur) and tetra-n-butylammonium bromide (TBAB) were from Merck (Darmstadt, Germany). Sodium(meta)-arsenite (≥99% purity) and Alcian blue were from Fluka Biochemika (Buchs, Germany). The cell-counting kit-8 (CCK-8®) was obtained from Dojindo Molecular Technologies (Munich, Germany) and the ICP-MS elemental standard (As, 1 mg L−1) from SPECTEC (Erding, Germany). AMP and ADP-ribose as well as hexadecane (HC 332), octadecane (HC 360) and tetracosane (HC 444) were obtained from Sigma (Deisenhofen, Germany). ATP and ADP were products of Gerbu (Gaiberg, Germany). Sodium hydroxide and hydrochloric acid were purchased from Grüssing (Filsum, Germany), acetonitrile from VWR (Darmstadt, Germany), dipotassium hydrogen phosphate was purchased from Roth (Karlsruhe, Germany), and 23-gauge needles were obtained from Braun (Melsungen, Germany). All other chemicals were of p.a. grade and were from Merck (Darmstadt, Germany) or Fluka Chemie (Buchs, Germany). Prof. Dr B. Epe (University of Mainz, Germany) kindly provided the Fpg protein. The urothelial cell line UROtsa was derived from a primary culture of a normal human urothelium through immortalisation with the SV-40 large T antigen. This cell line was kindly provided by Prof. M. Stýblo (University of North Carolina, USA). HepG2 cells were obtained from the European Collection of Cell Cultures (ECACC; number 85011430, Salisbury, UK).Synthesis and preparation of arsenic-containing hydrocarbons for cytotoxicity studies
The arsenic-containing hydrocarbons were synthesised and purified in Graz; full details have been reported elsewhere.25 In brief, iododimethylarsine was added to concentrated NaOH to form bis-(dimethylarsenic) oxide [(Me2As)2O], which was then heated with the appropriate 1-bromo-alkane. The product was extracted into chloroform, and crystallised from ethylacetate. The purity of the compounds was >99% as assessed by NMR spectroscopy and HPLC coupled with molecular (electrospray) and elemental (ICP-MS) mass spectrometry.A portion (ca. 15 mg) of the arsenolipid was transferred into a test tube and dissolved in ca. 10 mL of methanol. The arsenic purity of the compound was checked by HPLC/ICP-MS, and the precise arsenic concentration of this methanolic solution was determined by ICP-MS (performed on replicate portions of this solution that had been subjected to an acid digestion procedure). Replicates (10–15) of an appropriate aliquot of the methanolic solution of arsenolipid (each containing 250 μg of As or 3.33 μmol of compound) were then transferred into small screw-capped vials; methanol was allowed to evaporate and the vials were stored at 4 °C before use in the cytotoxicity studies.
Cell culture and incubation with arsenicals and alkanes
Cells were grown in culture dishes as a monolayer using MEM containing FCS (10%, v/v), penicillin (100 U mL−1) and streptomycin (100 μg mL−1) for UROtsa cells. For HepG2 cells MEM containing FCS (10%, v/v), penicillin (100 U mL−1) and streptomycin (100 μg mL−1) was used, which was supplemented with non-essential amino acids (1%, v/v). The cultures were incubated at 37 °C with 5% CO2 in the air with 100% humidity. For each experiment cells were seeded in a defined density (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per cm2). After 24 h, logarithmically growing cells were incubated with the respective compounds for 48 h.
000 cells per cm2). After 24 h, logarithmically growing cells were incubated with the respective compounds for 48 h.
Arsenical and alkane stock solutions (10 mM) were prepared in 100% EtOH and stored at 4 °C. For incubation the stock solution was diluted shortly before the experiment with EtOH. The EtOH concentration in all experiments was set to 0.5% for UROtsa cells and 1% for HepG2 cells. These concentrations did not induce any cytotoxic effects compared to untreated control cells (data not shown).
Cytotoxicity testing
The cytotoxicity of the arsenic-containing hydrocarbons and their respective non-arsenic-containing forms was elucidated by quantifying their effects on cell number, colony forming ability, lysosomal integrity as well as dehydrogenase activity.Cell number and colony forming ability
Cell number and colony forming ability testing were performed as described before.11 Briefly, after 48 h of incubation with the arsenicals or alkanes, UROtsa or HepG2 cells were washed with phosphate buffered saline (PBS) and trypsinised. Subsequently, cell number and cell volume were measured using an automatic cell counter (Casy TTC®, Roche Innovatis AG). To evaluate the impact of the compounds on colony forming ability in UROtsa cells, after cell counting of each sample, 500 cells per dish were seeded again and after 6–7 days, colonies were fixed with EtOH, stained with Giemsa (25% in EtOH), counted and calculated as percent of control. The colony forming ability test could not be carried out in HepG2 cells, since these cells cannot be fully singularised.Lysosomal integrity (neutral red uptake assay)
The neutral red uptake assay is based on the ability of viable cells to incorporate and bind the supravital dye neutral red in their lysosomes.26 UROtsa and HepG2 cells were cultured in 96-well culture plates and after 48 h of incubation with the respective compound the medium was replaced by neutral red (3-amino-7-dimethylamino-2-methylphenazine hydrochloride) containing medium (UROtsa: 66.7 mg L−1 and HepG2 55.6 mg L−1 neutral red in MEM). After dye loading (3 h, 37 °C), cells were washed with PBS containing 0.5% formaldehyde and the incorporated dye was solubilised in 100 μL of acidified EtOH solution (50% EtOH, 1% acetic acid in PBS). Finally, the absorbance in each well was measured using a plate reader (Tecan Infinite M200® PRO, Tecan Deutschland GmbH, Crailsheim, Germany) at 540 nm.Dehydrogenase activity (CCK-8 assay)
Cell viability was additionally assessed colorimetrically applying the cell-counting kit-8 (CCK-8).27 Briefly, UROtsa and HepG2 cells were cultured in 96-well culture plates and after 48 h of incubation with the respective compound, WST-8 (2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfo-phenyl)-2H-tetrazolium) solution was added and cells were incubated for 1 h. Absorbance in each well was determined using a plate reader (Tecan Infinite M200® PRO, Tecan Deutschland GmbH, Crailsheim, Germany) at 450 nm. By the activity of cellular dehydrogenases the water-soluble tetrazolium salt (WST-8) is reduced to a formazan dye. The amount of generated yellow-coloured formazan in the cells is directly proportional to the number of viable cells per well.LDH release
LDH release was observed in both the cell lysates and the dosing media as described before.28 Briefly, 40 μL of culture medium or 10 μL of cell lysates were mixed in a 96-well culture plate with reaction buffer (100 mM HEPES, 0.14 g L−1 NADH, 1.1 g L−1 sodium pyruvate, pH 7) to reach a total volume of 200 μL each. Absorbance was detected kinetically at 355 nm for every 1.5 min at 37 °C. LDH release was calculated as percentage of untreated control cells.Caspase-3 activity
Apoptosis was monitored by caspase-3 activity as previously reported.28 Briefly, after lysis of cells, the lysates were mixed with an equal amount of reaction buffer (50 mM PIPES, 10 mM EDTA, 0.5% CHAPS, 10 mM DTT, 80 μM DEVD-AFC) in a black 96-well plate. After 4 h of incubation at 37 °C, fluorescence of cleaved 7-amino-4-trifluoromethylcoumarin (AFC) was monitored (ex. 400 nm, em. 505 nm). Caspase-3 activity was normalised by the respective protein contents, which were quantified by bicinchoninic acid (BCA) assay.Cellular bioavailability of arsenic
Cellular bioavailability of the arsenic-containing hydrocarbons was studied after wet-ashing the cells (acid digestion) and measuring the arsenic content of the digest by inductively coupled plasma triple quadrupole mass spectrometry (ICP-MS/MS). Briefly, after 48 h of incubation with the arsenicals, cells were trypsinised, collected by centrifugation and washed with ice-cold PBS. For calculation of cellular arsenic concentrations, volumes of cells and nuclei were measured using an automatic cell counter (Casy TTC®, Roche Innovatis AG) in each sample; these measurements are based on non-invasive (dye-free) electrical current exclusion with signal evaluation via pulse area analysis. Mean (±SD) volumes of non-incubated UROtsa and HepG2 cells were 1.92 (±0.26) × 10−12 L and 1.98 (±0.29) × 10−12 L, respectively. All arsenicals showed no significant effects on cell volumes at non-cytotoxic concentrations. After incubation with the ashing mixture (65% HNO3–30% H2O2 (1/1, v/v)) at 95 °C for at least 12 h, samples were diluted with 0.15 N HNO3. Total arsenic was measured by ICP-MS/MS (Agilent 8800 ICP-QQQ, Agilent Technologies Deutschland GmbH, Böblingen, Germany) in the mass-shift mode using oxygen as a reaction gas to eliminate interferences. Further ICP-MS/MS conditions are listed in Table 1.Cellular distribution of arsenic
To assess cellular arsenic distribution after 48 h of incubation with the respective arsenic-containing hydrocarbons, cells were trypsinised, collected by centrifugation, and washed with ice-cold PBS. The cell number and cell volume were determined in each sample as described above. After centrifugation the cell pellet was lysed by addition of bi-distilled water and sonicated (15 s, 100%, 0.8 cycles). Subsequently, the membrane-associated parts were separated from the cytosol (and nuclei plasma) by centrifugation (5 min, 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 × g, 4 °C). ICP-MS/MS based analysis of total arsenic was carried out in the cytosol as well as in the wet-ashed membrane-containing fraction.
600 × g, 4 °C). ICP-MS/MS based analysis of total arsenic was carried out in the cytosol as well as in the wet-ashed membrane-containing fraction.
Alkaline unwinding
DNA strand breaks were quantified by the alkaline unwinding technique.27 Briefly, 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 HepG2 cells were seeded and after 24 h cells were incubated with the arsenicals for 48 h. Subsequently, the medium was removed, cells were washed with PBS and an alkaline solution (0.03 M NaOH, 0.02 M Na2HPO4, 0.9 M NaCl) was added. After neutralisation and sonication, separation of single- and double-stranded DNA was performed on 0.5 mL hydroxyapatite columns at 60 °C. Single- and double-stranded DNA were eluted with 1.5 mL of 0.15 M and 0.35 M potassium phosphate buffer, respectively. The DNA content of both fractions was determined by adding Hoechst 33
000 HepG2 cells were seeded and after 24 h cells were incubated with the arsenicals for 48 h. Subsequently, the medium was removed, cells were washed with PBS and an alkaline solution (0.03 M NaOH, 0.02 M Na2HPO4, 0.9 M NaCl) was added. After neutralisation and sonication, separation of single- and double-stranded DNA was performed on 0.5 mL hydroxyapatite columns at 60 °C. Single- and double-stranded DNA were eluted with 1.5 mL of 0.15 M and 0.35 M potassium phosphate buffer, respectively. The DNA content of both fractions was determined by adding Hoechst 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 258 dye to a final concentration of 0.77 μM to 1 mL of each sample and measuring the fluorescence using a microtiter fluorescence reader (Tecan Infinite M200® PRO, Tecan Deutschland GmbH, Crailsheim, Germany) at an excitation wavelength of 360 nm and an emission wavelength of 455 nm. DNA strand breaks were quantified by calibration with X-rays as described previously.29
258 dye to a final concentration of 0.77 μM to 1 mL of each sample and measuring the fluorescence using a microtiter fluorescence reader (Tecan Infinite M200® PRO, Tecan Deutschland GmbH, Crailsheim, Germany) at an excitation wavelength of 360 nm and an emission wavelength of 455 nm. DNA strand breaks were quantified by calibration with X-rays as described previously.29
Micronuclei formation
Micronuclei formation was studied as previously reported.30 Since our earlier studies indicated that several arsenicals interact with actin and/or the effect of cytochalasin B, we omitted the application of cytochalasin B.10 To ensure mitosis, cell proliferation was monitored by means of cell number quantification. An incubation time of 48 h was chosen, which in these cell lines is in accordance with around 2 cell cycles of untreated control cells. Briefly, UROtsa and HepG2 cells were seeded in 12-well plates on Alcian blue-coated glass coverslips. Cells were incubated with the respective arsenicals, fixed with an ice-cold fixation solution (90% methanol–10% PBS, −20 °C) for 10 min, stained with acridine orange (125 mg L−1 in PBS), and finally evaluated by fluorescence microscopy after coding of slides. Per coverslip, at least 1000 mononucleated cells were counted and categorised into mononucleated, binucleated, and multinucleated cells as well as cells with and without micronuclei.Level of energy related nucleotides
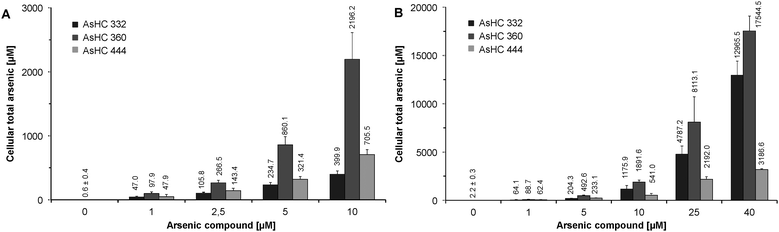
The cellular levels of energy related nucleotides were quantified as described previously31 with slight modifications. Shortly, incubated cells were trypsinised, the respective cell volumes were determined in each sample and cells were pelletised. Cell pellets were lysed by 300 μL of 0.5 M KOH, pulled 10 times through a 23-gauge needle and the extract was neutralised by adding 60 μL of phosphoric acid (10%). After centrifugation at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 630 × g (30 min, 4 °C), for separation of the nucleotides, 20 μL of the supernatant were injected into a LC system, consisting of an autosampler, a binary pump and a photodiode array detector (DAD) (Agilent 1200 series, Agilent Technologies Deutschland GmbH, Böblingen, Germany). The total adenosine nucleotides (TAN) and energy charge values (AEC) were calculated according to the following formulas for each sample:
630 × g (30 min, 4 °C), for separation of the nucleotides, 20 μL of the supernatant were injected into a LC system, consisting of an autosampler, a binary pump and a photodiode array detector (DAD) (Agilent 1200 series, Agilent Technologies Deutschland GmbH, Böblingen, Germany). The total adenosine nucleotides (TAN) and energy charge values (AEC) were calculated according to the following formulas for each sample:| TAN = [ATP] + [ADP] + [AMP] |
Statistical analysis
All experiments were carried out at least three times, each time on a different day. As indicated in the respective figure captions, from the raw data the mean standard deviation (SD) was calculated and a statistical analysis was performed by using the ANOVA-OneWay-test. Significance levels are *p < 0.05, **p < 0.01 and ***p < 0.001.Results
Cytotoxicity
First, to get an idea about the in vitro toxicological profile of the respective arsenolipids, several cytotoxicity markers were examined after 48 h of incubation with the three arsenic-containing hydrocarbons (AsHC 332, AsHC 360 and AsHC 444). In parallel arsenite (iAsIII) was studied as the toxic reference arsenical. Thus, the effects on cell number and colony forming ability as well as on lysosomal integrity and dehydrogenase activity were quantified.In UROtsa cells, cytotoxicity caused by the arsenic-containing hydrocarbons was in the same concentration range as that shown by iAsIII (Fig. 2). The two longer C-chain arsenic-containing hydrocarbons (AsHC 360 and AsHC 444) were comparable in their toxic behaviour, whereas the smaller AsHC 332 was less cytotoxic in all investigated endpoints than AsHC 360 and AsHC 444. iAsIII exerted slightly stronger effects than did the arsenolipids regarding the endpoints, cell number and colony forming ability, but showed weaker effects for the viability markers, lysosomal integrity and dehydrogenase activity.
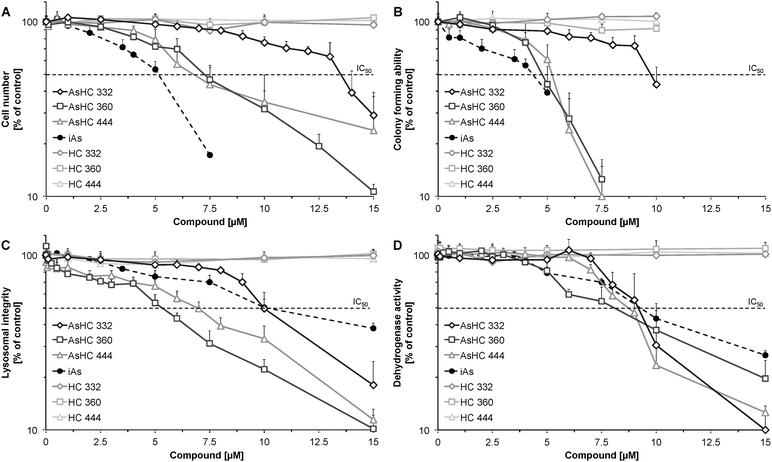
HepG2 cells were less sensitive towards all applied arsenicals than UROtsa cells (Fig. 3); IC50 values of the arsenic-containing hydrocarbons were around 5-fold higher as compared to the respective IC50 values in UROtsa cells (Table 2). The toxicity order of the arsenolipids was similar in UROtsa and HepG2 cells, with AsHC 360 being the most and AsHC 332 being the less cytotoxic arsenolipid applied. Regarding all endpoints, iAsIII was similar or less cytotoxic as compared to the respective arsenic-containing hydrocarbons.
| 48 h incubation | Cell number | Colony forming ability | Lysosomal integrity | Dehydrogenase activity | |
|---|---|---|---|---|---|
| AsHC | UROtsa | 13.5 | 9.8 | 10.0 | 9.2 |
| 332 | HepG2 | 17 | — | 17 | 52 |
| AsHC | UROtsa | 7.4 | 4.8 | 5.2 | 8.0 |
| 360 | HepG2 | 8 | — | 21 | 30 |
| AsHC | UROtsa | 7.0 | 5.2 | 7.0 | 8.8 |
| 444 | HepG2 | 21 | — | 40 | 63 |
| Arsenite | UROtsa | 5.2 | 4.3 | 10.1 | 9.2 |
| HepG2 | 17 | — | 44 | 38 |
To verify that the toxic effects are associated with the dimethylarsinoyl group of the arsenic-containing hydrocarbons the corresponding hydrocarbons (HC 332, HC 360, and HC 444) were studied in parallel. Cytotoxic effects were not observed in the applied respective concentrations of the alkanes, indicating that the dimethylarsinoyl group of the molecules is likely to be responsible for the cytotoxicity.
Cellular bioavailability and distribution
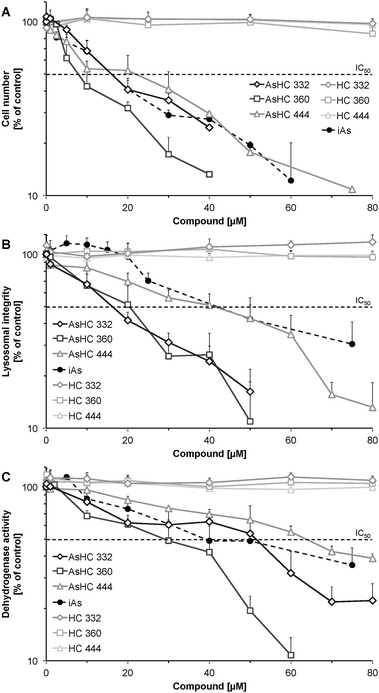
The cellular arsenic concentrations were determined after 48 h of incubation with the three arsenic-containing hydrocarbons in both UROtsa and HepG2 cells. All three arsenic-containing hydrocarbons were strongly bioavailable in both UROtsa and HepG2 cells (Fig. 4). In UROtsa cells after incubation with a low sub-cytotoxic concentration (1 μM) of the arsenolipids, cellular arsenic accumulated by a factor of up to 100 in relation to the incubated extracellular arsenic concentration. The most cytotoxic arsenolipid AsHC 360 exerted the highest cellular bioavailability, reaching a cellular accumulation factor of 200. Similar accumulation behaviour was observed in HepG2 cells. For AsHC 360 even an accumulation of more than 400-fold was observable.Although iAsIII was cellularly bioavailable as well, cellular accumulation was much lower in both cell lines. Thus, after incubation with iAsIII, cellular arsenic content was merely up to 10-fold higher than the respective extracellular incubation concentration (Table 3).
| Incubation concentration [μM] | 1 | 5 | 10 | 25 |
|---|---|---|---|---|
| UROtsa | 10.3 ± 1.7 | 53.0 ± 10.9 | — | — |
| HepG2 | 11.5 ± 6.1 | 34.9 ± 10.9 | 57.7 ± 10.9 | 547 ± 60 |
Distribution analysis demonstrated that around 30–56% of the total cellular arsenic is localised in membrane-associated parts (Table 4) after incubation with the arsenolipids. When taking into account that the membrane fraction captures only a small portion of the cell, it becomes clear that the absolute arsenic concentration in the membrane fraction is much higher than in the cytosol.
Formation of micronuclei and induction of DNA damage
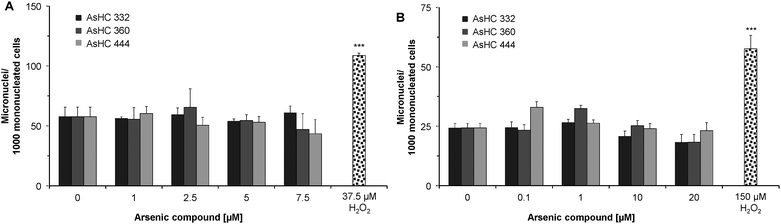
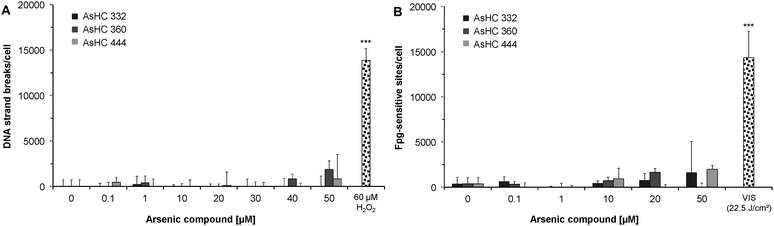
Genotoxicity of three arsenic-containing hydrocarbons was assessed at both the chromosomal and DNA levels, by quantifying the formation of micronuclei, DNA strand breaks and oxidative base modifications.In both cell lines the arsenolipids increased neither the number of micronuclei (Fig. 5A and B), nor the amount of bi- or multinucleated cells (data not shown). Additionally the arsenolipids did not induce DNA strand breaks (Fig. 6A) and Fpg-sensitive sites (Fig. 6B) in HepG2 cells.
Lactate dehydrogenase (LDH) release and caspase-3 activity
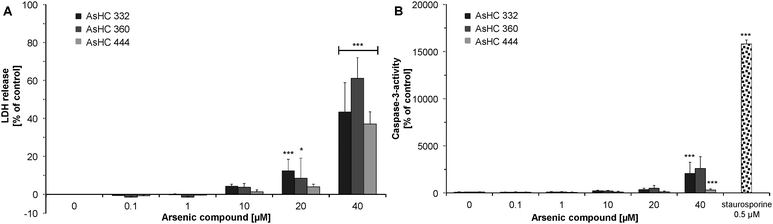
LDH release was monitored in HepG2 cells to assess the effects of the arsenicals on cell membrane integrity. In comparison to other studied endpoints, LDH release was the most insensitive viability marker, showing no significant effects up to 10 μM of the respective arsenolipids. After incubation with 40 μM of the arsenicals, a strong LDH release was observable for all three arsenic-containing hydrocarbons (Fig. 7A). This indicates that in the highly cytotoxic concentration range, the arsenolipids also disturb cell membrane integrity.Apoptosis was monitored via caspase-3 activity in HepG2 cells. AsHC 360 did not show any significant caspase-3 activity up to an incubation concentration of 40 μM (Fig. 7B). AsHC 332 and AsHC 444 exerted a significant increase of capase-3 activity only after incubation with 40 μM. These data strongly suggest that the respective arsenolipids do not cause apoptotic cell death in the applied concentration range. This indication is supported by the fact that no apoptotic bodies were visible in the microscopic slides prepared for the micronuclei test after incubation with the arsenic-containing hydrocarbons (data not shown).
Level of energy related nucleotides
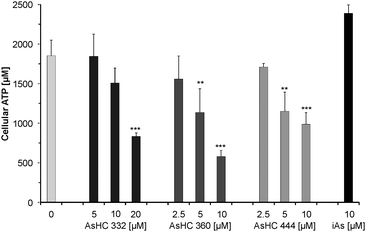
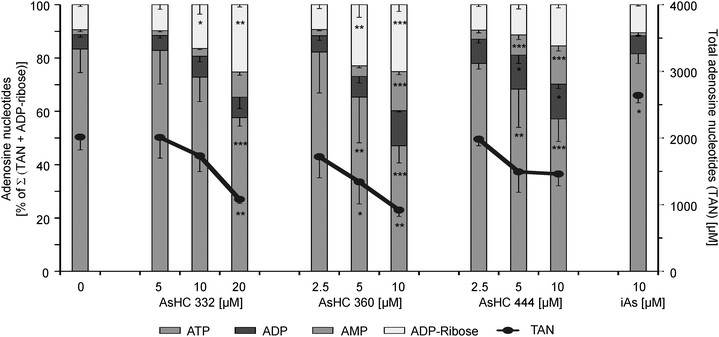
The observed ATP and total adenosine nucleotide (TAN) levels of the investigated HepG2 cells are in the range of published data. In tumour cells the ATP concentration is 3134 ± 2135 μM32 and in the present work the ATP level of HepG2 cells was 1620 ± 50 μM (Fig. 8). This represents around 90% of the TAN. This result is comparable with the measured ATP levels in A549 cells (1595 ± 291 μM, 90% of the TAN) in a former study.31 The energy charge value (AEC) of untreated HepG2 cells was calculated to be 0.95 (data not shown), which indicates healthy cells, where the AEC is normally between 0.7 and 1.0.33Incubation with slightly cytotoxic effects of the respective arsenolipids caused a significant decrease of the TAN levels (Fig. 9). The cellular ATP levels were strongly affected, leaving only 44.9%, 31.2% and 53.3% of cellular ATP in the case of incubation with 20 μM of AsHC 332 or 10 μM of AsHC 360 or AsHC 444, respectively (Fig. 8). Corresponding to the decrease of ATP levels, the cellular levels of AMP, ADP and ADP-ribose increased (Fig. 9). Compared to control cells the amount of ADP and ADP-ribose is up to 2-fold higher and the AMP concentration is even around 3 to 6-fold higher in cells incubated with the arsenolipids. This indicates that ATP recycling as well as ATP synthesis is disturbed.
Changes in the distribution of the levels of the adenosine nucleotides are also evident for the AEC values, which were decreased by the arsenolipids. Thus, for 20 μM of AsHC 332 the AEC value was 0.82, and for 10 μM of AsHC 360 and AsHC 444 the values were 0.72 and 0.75, respectively, (data not shown).
In contrast to the arsenolipids, iAsIII did not significantly decrease the cellular ATP level up to an incubation concentration of 10 μM. These data suggest that iAsIII causes cell death in vitro via a different mode of action than the arsenic-containing hydrocarbons.
Discussion
This study investigated for the first time the cellular toxicity and bioavailability of three food-relevant arsenic-containing hydrocarbons. Arsenite was studied in parallel as the toxic arsenic reference species, to facilitate a first in vitro based risk assessment for this class of arsenolipids.In both cultured human bladder (UROtsa) and liver (HepG2) cells, the three arsenic-containing hydrocarbons caused significant cytotoxicity, which occurred in the same concentration range as that observed after incubation with iAsIII. In comparison to other seafood-relevant arsenicals in vitro, the arsenolipids are at least 600-fold more toxic than a glycerol arsenosugar,34,35 and about 20 to 25-fold more toxic than their major metabolite DMAV.30
This high cellular toxicity of the arsenolipids is likely to result at least partly from their cellular bioavailability. The highly lipophilic arsenic-containing hydrocarbons seem to be able to easily pass the cell membranes, presumably by passive diffusion, enter the cell and accumulate inside the cell especially in lipophilic membranes. This assumption was supported by the cellular distribution studies, which indicated a strong affinity of the arsenolipids to the membrane-associated cellular fraction.
From a structural point of view, the arsenic-containing hydrocarbons are amphiphilic, consisting of a hydrophobic hydrocarbon tail and a hydrophilic dimethylarsinoyl head group. Therefore, these molecules might interact with bio-membranes and attach to these membranes comparable to phospholipids. In this context it has already been discussed that AsPL might be used in membrane chemistry by algae.19,36
Furthermore, recently it has been demonstrated that phytoplankton can use non-phosphorus lipids to form membrane lipids.37 In the case of phosphorus scarcity in oceanic waters, nitrogen or sulfur instead of phosphorus was used to build-up membrane lipids by the investigated algae. Arsenic is a member of the same group in the periodic table as phosphorus, and hence it is similar in some of its physical and chemical characteristics. Possibly, arsenic may also be used to form membrane lipids by algae living under phosphorus-deficient conditions, especially when non-selective enzymes are involved.
To elucidate the toxic modes of action of the arsenic-containing hydrocarbons, their genotoxic potential was investigated. In previous studies an increase in micronuclei induction by iAsIII was observed (e.g.ref. 10, 38 and 39). Nevertheless, the three arsenic-containing hydrocarbons did not increase the number of micronuclei in both UROtsa and HepG2 cells. Likewise, the frequency of bi- and multinucleated cells was not affected by the arsenolipids. Whereas iAsIII has been shown before to generate DNA lesions, including Fpg-sensitive sites (e.g.ref. 40 and 41), the arsenolipids failed to increase the cellular amount of DNA strand breaks and Fpg-sensitive sites. These data suggest that the observed cellular toxicity of the arsenic-containing hydrocarbons does not result from a genotoxic mode of action.
Further studies also excluded the possibility that cell death was triggered by apoptosis or loss of cell membrane integrity. These endpoints were affected by the arsenic-containing hydrocarbons only at highly cytotoxic concentrations.
Lysosomal integrity has been the most sensitive viability marker investigated after arsenolipid exposure in this study. It is well known that lysosomal integrity strongly depends on the cellular ATP level. Thus, ATP is necessary to maintain the membrane potential of these organelles, which means they have a pH value of approximately 4.5–5.26 Accordingly, all three applied arsenic-containing hydrocarbons caused a concentration dependent decrease in the cellular amount of ATP. Besides its role as an energy carrier, ATP is also very important in signalling pathways and development and regeneration processes.42 Consequently, a low ATP level can cause a number of cellular disorders, which are likely to contribute to the observed arsenolipid induced cell death in the present study. A possible underlying mechanism for the decrease in cellular ATP levels is that the arsenolipids attack mitochondrial membranes, thereby disturbing mitochondrial function and cellular ATP production. This hypothesis will be tested in our future studies.
Conclusion
Whereas arsenobetaine and arsenosugars have been shown to exert no or only very low toxicity in cultured cells,7,8,30,35 the three arsenic-containing hydrocarbons showed a strong cytotoxic potential in the low μM concentration range, which is comparable to the cytotoxic potential found for arsenite. However, the toxic modes of action seem to differ between arsenite and the arsenic-containing hydrocarbons highlighting the need for further in vitro studies to understand the toxic mode of action of this potentially highly toxic class of organic arsenicals.To finally assess the risk to human health, toxicity studies in experimental animals should be carried out urgently.
Acknowledgements
This work was supported by the DFG grant number SCHW903/4-1, the Austrian Science Fund (FWF), project number I550-N17, and the Graduate School of Chemistry (WWU Münster, Germany).References
- K. A. Francesconi, Arsenic species in seafood: Origin and human health implications, Pure Appl. Chem., 2010, 85, 373–381 Search PubMed.
- K. Straif, L. Benbrahim-Tallaa, R. Baan, Y. Grosse, B. Secretan, F. El Ghissassi, V. Bouvard, N. Guha, C. Freeman, L. Galichet and V. Cogliano, A review of human carcinogens: metals, arsenic, dusts, and fibres, Lancet Oncol., 2009, 10, 453–454 CrossRef.
- EFSA, EFSA Panel on Contaminants in the Food Chain (CONTAM); Scientific Opinion on Arsenic in Food, EFSA J., 2009, 7, 1351–1355 Search PubMed.
- M. S. Taleshi, J. S. Edmonds, W. Goessler, M. J. Ruiz-Chancho, G. Raber, K. B. Jensen and K. A. Francesconi, Arsenic-containing lipids are natural constituents of sashimi tuna, Environ. Sci. Technol., 2010, 44, 1478–1483, DOI:10.1021/es9030358.
- V. Sele, J. J. Sloth, A. K. Lundebye, E. H. Larsen, M. H. G. Berntssen and H. Amlund, Arsenolipids in marine oils and fats: A review of occurrence, chemistry and future research needs, Food Chem., 2012, 133, 618–630, DOI:10.1016/j.foodchem.2012.02.004.
- E. Schmeisser, W. Goessler and K. A. Francesconi, Human metabolism of arsenolipids present in cod liver, Anal. Bioanal. Chem., 2006, 385, 367–376, DOI:10.1007/s00216-006-0401-x.
- T. Kaise, S. Watanabe and K. Itoh, The Acute Toxicity of Arsenobetaine, Chemosphere, 1985, 14, 1327–1332, DOI:10.1016/0045-6535(85)90153-5.
- C. Newcombe, A. Raab, P. N. Williams, C. Deacon, P. I. Haris, A. A. Meharg and J. Feldmann, Accumulation or production of arsenobetaine in humans?, J. Environ. Monit., 2010, 12, 832–837, 10.1039/b921588c.
- E. Schmeisser, A. Rumpler, M. Kollroser, G. Rechberger, W. Goessler and K. A. Francesconi, Arsenic fatty acids are human urinary metabolites of arsenolipids present in cod liver, Angew. Chem., Int. Ed., 2006, 45, 150–154, DOI:10.1002/anie.200502706.
- M. Bartel, F. Ebert, L. Leffers, U. Karst and T. Schwerdtle, Toxicological Characterization of the Inorganic and Organic Arsenic Metabolite Thio-DMA in Cultured Human Lung Cells, J. Toxicol., 2011, 2011, 1–9, DOI:10.1155/2011/373141.
- F. Ebert, A. Weiss, M. Bultemeyer, I. Hamann, A. Hartwig and T. Schwerdtle, Arsenicals affect base excision repair by several mechanisms, Mutat. Res., 2011, 715, 32–41, DOI:10.1016/j.mrfmmm.2011.07.004.
- M. J. Mass, A. Tennant, B. C. Roop, W. R. Cullen, M. Styblo, D. J. Thomas and A. D. Kligerman, Methylated trivalent arsenic species are genotoxic, Chem. Res. Toxicol., 2001, 14, 355–361 CrossRef CAS PubMed.
- US-EPA, Advisory on EPAs assessment of carcinogenic effects of organic and inorganic arsenic, U. S. E. P. A. S. A. Board, Washington, DC, USA, EPA-SAB-07-008, 2007 Search PubMed.
- IARC, A review of human carcinogens. Part C: Arsenic, metals, fibres, and dusts, IARC Monographs, 2012, 196–211.
- G. Lunde, Analysis of arsenic in marine oils by neutron activation. Evidence of arseno organic compounds, J. Am. Oil Chem. Soc., 1968, 45, 331–332 CrossRef CAS.
- M. Morita and Y. Shibata, Isolation and identification of arseno-lipid from a brown alga, Chemosphere, 1988, 17, 1147–1152 CrossRef CAS.
- M. S. Taleshi, K. B. Jensen, G. Raber, J. S. Edmonds, H. Gunnlaugsdottir and K. A. Francesconi, Arsenic-containing hydrocarbons: natural compounds in oil from the fish capelin, Mallotus villosus, Chem. Commun., 2008, 4706–4707, 10.1039/b808049f.
- A. Rumpler, J. S. Edmonds, M. Katsu, K. B. Jensen, W. Goessler, G. Raber, H. Gunnlaugsdottir and K. A. Francesconi, Arsenic-containing long-chain fatty acids in cod-liver oil: a result of biosynthetic infidelity?, Angew. Chem., Int. Ed., 2008, 47, 2665–2667, DOI:10.1002/anie.200705405.
- S. Garcia-Salgado, G. Raber, R. Raml, C. Magnes and K. A. Francesconi, Arsenosugar phospholipids and arsenic hydrocarbons in two species of brown macroalgae, Environ. Chem., 2012, 9, 63–66 CrossRef CAS.
- K. O. Amayo, A. Petursdottir, C. Newcombe, H. Gunnlaugsdottir, A. Raab, E. M. Krupp and J. Feldmann, Identification and quantification of arsenolipids using reversed-phase HPLC coupled simultaneously to high-resolution ICP-MS and high-resolution electrospray MS without species-specific standards, Anal. Chem., 2011, 83, 3589–3595, DOI:10.1021/ac2005873.
- K. O. Amayo, A. Raab, E. M. Krupp and J. Feldmann, Novel identification of lipid-soluble arsenic compounds using chemical derivatisations in conjunction with RP-HPLC-ICPMS/ESMS, Anal. Chem., 2013, 85, 9321–9327, DOI:10.1021/ac4020935.
- U. Arroyo-Abad, S. Lischka, C. Piechotta, J. Mattusch and T. Reemtsma, Determination and identification of hydrophilic and hydrophobic arsenic species in methanol extract of fresh cod liver by RP-HPLC with simultaneous ICP-MS and ESI-Q-TOF-MS detection, Food Chem., 2013, 141, 3093–3102, DOI:10.1016/j.foodchem.2013.05.152.
- U. Arroyo-Abad, J. Mattusch, S. Mothes, M. Moder, R. Wennrich, M. P. Elizalde-Gonzalez and F. M. Matysik, Detection of arsenic-containing hydrocarbons in canned cod liver tissue, Talanta, 2010, 82, 38–43, DOI:10.1016/j.talanta.2010.03.054.
- S. Lischka, U. Arroyo-Abad, J. Mattusch, A. Kuhn and C. Piechotta, The high diversity of arsenolipids in herring fillet (Clupea harengus), Talanta, 2013, 110, 144–152, DOI:10.1016/j.talanta.2013.02.051.
- M. S. Taleshi, R. K. Seidler-Egdal, K. B. Jensen, T. Schwerdtle and K. A. Francesconi, Synthesis and Characterisation of Arsenolipids: Naturally Occurring Arsenic Compounds in Fish and Algae, Organometallics, 2014, 33, 1397–1403, DOI:10.1021/om4011092.
- G. Repetto, A. del Peso and J. L. Zurita, Neutral red uptake assay for the estimation of cell viability/cytotoxicity, Nat. Protoc., 2008, 3, 1125–1131, DOI:10.1038/nprot.2008.75.
- J. Bornhorst, F. Ebert, A. Hartwig, B. Michalke and T. Schwerdtle, Manganese inhibits poly(ADP-ribosyl)ation in human cells: a possible mechanism behind manganese-induced toxicity?, J. Environ. Monit., 2010, 12, 2062–2069, 10.1039/c0em00252f.
- F. Ebert, L. Leffers, T. Weber, S. Berndt, A. Mangerich, S. Beneke, A. Burkle and T. Schwerdtle, Toxicological properties of the thiolated inorganic arsenic and arsenosugar metabolite thio-dimethylarsinic acid in human bladder cells, J. Trace Elem. Med. Biol., 2013 DOI:10.1016/j.jtemb.2013.06.004.
- A. Hartwig, U. D. Groblinghoff, D. Beyersmann, A. T. Natarajan, R. Filon and L. H. Mullenders, Interaction of arsenic(III) with nucleotide excision repair in UV-irradiated human fibroblasts, Carcinogenesis, 1997, 18, 399–405 CrossRef CAS PubMed.
- L. Leffers, F. Ebert, M. S. Taleshi, K. A. Francesconi and T. Schwerdtle, In vitro toxicological characterization of two arsenosugars and their metabolites, Mol. Nutr. Food Res., 2013, 57, 1270–1282, DOI:10.1002/mnfr.201200821.
- J. Bornhorst, F. Ebert, H. Lohren, H. U. Humpf, U. Karst and T. Schwerdtle, Effects of manganese and arsenic species on the level of energy related nucleotides in human cells, Metallomics, 2012, 4, 297–306, 10.1039/c2mt00164k.
- T. W. Traut, Physiological concentrations of purines and pyrimidines, Mol. Cell. Biochem., 1994, 140, 1–22 CrossRef CAS.
- M. S. Yang, L. C. Yu and R. C. Gupta, Analysis of changes in energy and redox states in HepG2 hepatoma and C6 glioma cells upon exposure to cadmium, Toxicology, 2004, 201, 105–113, DOI:10.1016/j.tox.2004.04.007.
- P. Andrewes, D. M. Demarini, K. Funasaka, K. Wallace, V. W. Lai, H. Sun, W. R. Cullen and K. T. Kitchin, Do arsenosugars pose a risk to human health? The comparative toxicities of a trivalent and pentavalent arsenosugar, Environ. Sci. Technol., 2004, 38, 4140–4148 CrossRef CAS.
- L. Leffers, M. Unterberg, M. Bartel, C. Hoppe, I. Pieper, J. Stertmann, F. Ebert, H. U. Humpf and T. Schwerdtle, In vitro toxicological characterisation of the S-containing arsenic metabolites thio-dimethylarsinic acid and dimethylarsinic glutathione, Toxicology, 2013, 305, 109–119, DOI:10.1016/j.tox.2013.01.007.
- R. V. Cooney, R. O. Mumma and A. A. Benson, Arsoniumphospholipid in algae, Proc. Natl. Acad. Sci. U. S. A., 1978, 75, 4262–4264 CrossRef CAS.
- B. A. Van Mooy, H. F. Fredricks, B. E. Pedler, S. T. Dyhrman, D. M. Karl, M. Koblizek, M. W. Lomas, T. J. Mincer, L. R. Moore, T. Moutin, M. S. Rappe and E. A. Webb, Phytoplankton in the ocean use non-phosphorus lipids in response to phosphorus scarcity, Nature, 2009, 458, 69–72, DOI:10.1038/nature07659.
- T. W. Gebel, Genotoxicity of arsenical compounds, Int. J. Hyg. Environ. Health, 2001, 203, 249–262, DOI:10.1078/S1438-4639(04)70036-X.
- T. S. Wang and H. Huang, Active oxygen species are involved in the induction of micronuclei by arsenite in XRS-5 cells, Mutagenesis, 1994, 9, 253–257 CrossRef CAS.
- T. Schwerdtle, I. Walter, I. Mackiw and A. Hartwig, Induction of oxidative DNA damage by arsenite and its trivalent and pentavalent methylated metabolites in cultured human cells and isolated DNA, Carcinogenesis, 2003, 24, 967–974 CrossRef CAS PubMed.
- T. S. Wang, T. Y. Hsu, C. H. Chung, A. S. Wang, D. T. Bau and K. Y. Jan, Arsenite induces oxidative DNA adducts and DNA-protein cross-links in mammalian cells, Free Radical Biol. Med., 2001, 31, 321–330 CrossRef CAS.
- G. Burnstock, Historical review: ATP as a neurotransmitter, Trends Pharmacol. Sci., 2006, 27, 166–176, DOI:10.1016/j.tips.2006.01.005.
| This journal is © The Royal Society of Chemistry 2014 |