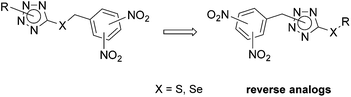
Tetrazole regioisomers in the development of nitro group-containing antitubercular agents†
Galina Karabanovich a, Jaroslav Roh *a, Ondřej Soukup b, Ivona Pávková c, Markéta Pasdiorová b, Vojtěch Tambor b, Jiřina Stolaříková d, Marcela Vejsová a, Kateřina Vávrová a, Věra Klimešová a and Alexandr Hrabálek a
aCharles University in Prague, Faculty of Pharmacy in Hradec Králové, Heyrovského 1203, 50005 Hradec Králové, Czech Republic. E-mail: jaroslav.roh@faf.cuni.cz
bBiomedical Research Center, University Hospital Hradec Králové, Sokolská 581, 50005 Hradec Králové, Czech Republic
cDepartment of Molecular Pathology and Biology, Faculty of Military Health Sciences, University of Defence, Třebešská 1575, 50005 Hradec Králové, Czech Republic
dRegional Institute of Public Health, Department of Bacteriology and Mycology, Partyzánské náměstí 7, 70200 Ostrava, Czech Republic
First published on 25th September 2014
Abstract
Tetrazole derivatives containing nitro substituents have been identified as promising antitubercular agents. In this study, the antitubercular potency, selectivity and toxicity of tetrazole 1,5- and 2,5-regioisomers were examined. We prepared a series of 1- and 2-alkyl-5-benzylsulfanyl-2H-tetrazoles and their selenium analogs with various nitro group substitutions. These 1,5- and 2,5-regioisomers were isolated and unambiguously identified using 1H and/or 13C NMR. Among the prepared compounds, 1- and 2-alkyl-5-[(3,5-dinitrobenzyl)sulfanyl]-2H-tetrazole derivatives and their selenium bioisosteres showed the highest antimycobacterial activity, with minimal inhibitory concentration (MIC) values of approximately 1 μM (0.37–0.46 μg mL−1) against Mycobacterium tuberculosis CNCTC My 331/88. The 2-alkyl regioisomers exhibited consistently higher antimycobacterial activity and lower in vitro toxicity against a mammalian cell line compared to the 1-alkyl isomers. The antimycobacterial activity of the 2-alkyl regioisomers was less influenced by the type of alkyl substituent in contrast to 1-alkyl isomers. Furthermore, the 3,5-dinitrobenzyl moiety per se is not the carrier of mutagenicity. These findings encourage further optimization of the 2-alkyl chain to improve the pharmacokinetic properties and toxicity of 2-alkyl-5-[(3,5-dinitrobenzyl)sulfanyl]-2H-tetrazole lead compounds.
Introduction
Tuberculosis (TB) is a widespread infectious disease and remains a serious global problem that takes millions of lives each year.1 The emergence and distribution of multi-drug resistant (MDR-TB) and extensively drug-resistant (XDR-TB) strains of Mycobacterium tuberculosis (M. tb) have become the biggest challenges in treatment with current anti-TB drugs. Current anti-TB drugs also suffer from low tolerability or adverse effects. Moreover, TB frequently occurs in HIV/AIDS patients who have a further reduced response to TB treatment.This establishes the need for the discovery of new highly efficient antimycobacterial agents (for recent reviews, see ref. 2–5). Recently, several nitro group-containing anti-TB agents were developed and two nitroimidazole-based compounds, PA-8246 and OPC-67683 (delamanid),7 are undergoing clinical trials.8 Another promising group of nitro group-containing anti-TB agents are dinitrobenzamides9 and benzothiazinones;10 both of which are inhibitors of decaprenyl-phosphoribose epimerase (DprE1), an essential enzyme involved in arabinan biosynthesis.11 Piperazinobenzothiazinone PBTZ 169, a benzothiazinone-derivative, is currently undergoing preclinical development.12 In our previous work, we found that 2-(dinitrobenzylsulfanyl)benzazoles 1 exhibited high in vitro antimycobacterial activities with minimal inhibitory concentrations (MICs) from 2 to 8 μM against M. tb (Fig. 1).13–16 Hence, to continue this study, we prepared a series of 1-alkyl/aryl-5-(dinitrobenzylsulfanyl)-1H-tetrazoles 2 with 1-substituted tetrazole in place of the original benzazole moiety (Fig. 1). The antimycobacterial activities of these compounds confirmed that the presence of two nitro groups on the benzylsulfanyl moiety is vital for increased antimycobacterial activity. 3,5-Dinitro substituted tetrazole derivatives of series 2 exhibited higher activities compared to the 2,4-dinitro analogs, with MIC values of 1 μM (0.36–0.44 μg mL−1) against M. tb, i.e., values equivalent to the first-line anti-TB drug isoniazid, and 0.25–1 μM against six MDR M. tb strains and with no cross-resistance with common anti-TB drugs. Moreover, the series of compounds 2 were highly selective for mycobacteria, because they exhibited no antibacterial or antifungal activity and low toxicity on selected mammalian cell lines. A structure–activity relationship study showed that the isosteric replacement of sulfur for the selenium or oxygen atom had no significant effect on the antimycobacterial activity of these compounds. In addition, twenty-three substituents at position 1 of the tetrazole were studied, and these appeared to influence the antimycobacterial activity of the compound through their effects on its lipophilicity.17
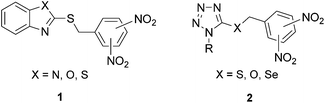 | ||
| Fig. 1 Dinitrobenzyl-bearing benzazole and tetrazole derivatives with high and selective antimycobacterial activity. | ||
As 5-substituted tetrazole forms 1H- and 2H-tautomers (3 and 4, Fig. 2),18–20 1,5- and 2,5-disubstituted tetrazole isomers can be recognized. Several studies have indicated that the biological properties of 1,5- and 2,5-tetrazole regioisomers differ, particularly in their effective concentration levels.21–23 4-Phenyl-1-(1H-tetrazol-1-yl)-2-butanone (5, Fig. 3), an inhibitor of heme oxygenase-1 (HO-1), displayed an IC50 value of 2.6 μM, while its 2H-tetrazol-2-yl analog exhibited greater than 3.5 times weaker inhibitory activity.24 1-Methyl derivatives of cephalosporin 6 displayed a two-fold increase in antibacterial activity against both Gram-positive and Gram-negative bacteria compared to the 2-methyl isomers.25 Conversely, 2,5-disubstituted tetrazoles 7 were efficient Pseudomonas aeruginosa quorum-sensing inhibitors, whereas 1,5-disubstituted analogs had low inhibitory activity.26N-Aryl-N′-tetrazole-substituted ureas 8 containing a long carbon chain in position 2 of the tetrazole ring displayed 10-fold higher acyl-CoA:cholesterol O-acyltransferase inhibition compared to the 1-regioisomers (Fig. 3).27
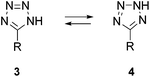 | ||
| Fig. 2 1H- and 2H-tautomers of 5-substituted tetrazoles. | ||
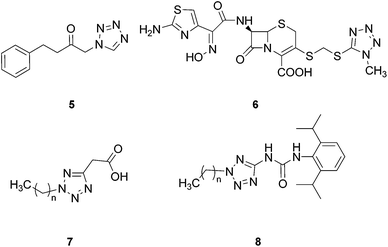 | ||
| Fig. 3 Examples of 1,5- and 2,5-disubstituted tetrazole-based compounds with regioisomer-dependent activity. | ||
In this work, we focused on the tetrazole-based lead compounds 2 and studied how the position of substituent R on tetrazole influenced their biological properties, specifically, antimycobacterial activity. Moreover, their reverse analogs, with a dinitrobenzyl moiety on tetrazole and with substituent R on sulfur/selenium in position 5 of tetrazole, were prepared to determine the optimal position of the crucial dinitrobenzyl fragment. We also examined the effect of isosteric replacement of sulfur for selenium on the biological properties of the target substances (Fig. 4).
Results and discussion
Chemistry
Synthesis of 1-alkyl-5-(alkylselanyl)-1H-tetrazoles (15–19) and their 2-regioisomers (20–24) was conducted according to Scheme 1. The starting alkyl selenocyanates (9–13) were obtained by the reaction of the corresponding alkyl halides with a slight excess of potassium selenocyanate in THF or DMF. Subsequently, they were converted into 5-(alkylselanyl)-1H-tetrazolates by the reaction with sodium azide and triethylammonium chloride in toluene at an elevated temperature.28,29 For the preparation of 5-[(2,4-dinitrobenzyl)selanyl]-1H-tetrazolate, the reaction mixture was stirred in THF at room temperature overnight to avoid the precipitation of selenium, which was observed using the above-mentioned procedure in toluene. 5-[(3,5-Dinitrobenzyl)selanyl]-1H-tetrazolate was sensitive to acidification, and the corresponding tetrazole 14 could only be prepared in low yield; therefore, we alkylated selanyltetrazolates in situ under the conditions of phase-transfer catalysis in the presence of tetrabutylammonium bromide (TBAB). Expectedly, the alkylation of 5-(alkylselanyl)-1H-tetrazolates led to the formation of both regioisomers,20 1-alkyl-5-(alkylselanyl)-1H-tetrazoles (15–19) and 2-alkyl-5-(alkylselanyl)-2H-tetrazoles (20–24), in moderate yields. 1- (15–19) and 2-isomers (20–24) of the target selanyltetrazoles were separated and purified by column chromatography. The ratios of 1- and 2-regioisomers ranged from 1 : 1 to 1 : 4.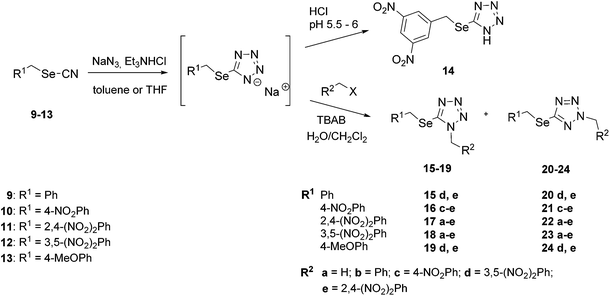 | ||
| Scheme 1 Synthesis of 1-alkyl- and 2-alkyl-5-(alkylselanyl)-2H-tetrazoles. | ||
The alkylation was preferentially directed to position 2, which is likely due to steric hindrance of the alkylselanyl substituent in position 5 of tetrazole. The 1,5- and 2,5-isomeric series were unambiguously identified according to the 1H and 13C NMR chemical shifts of the tetrazole carbon and the nitrogen-bound methylene group. These signals of 2-alkyl-5-(alkylselanyl)-2H-tetrazoles were observed downfield compared to the 1-isomers (Table 1). Correlations between methylene hydrogens in 1,5-isomer 19d in 1D NOESY experiments and the absence of such correlations in 2,5-isomer 24d confirmed their regioisomeric identities (see ESI†). Furthermore, all 2-alkyl-5-(alkylselanyl)-2H-tetrazoles had lower retention (higher values of Rf) on silica gel compared to their 1-isomers, indicating that the 2-isomers were less polar. In contrast to the 1-alkyl-5-(alkylselanyl)-1H-tetrazole regioisomers,30 a report on 2-alkyl regioisomers has not yet been published. Nevertheless, our results are in agreement with previously published differences between NMR shifts of 1,5- and 2,5-disubstituted tetrazoles.31,32
| 1-/2-Isomers | R1-CH2Se | R2-CH2N | 1-Isomer | 2-Isomer | ||
|---|---|---|---|---|---|---|
| Ctetr | R2-CH2N1 | Ctetr | R2-CH2N2 | |||
| a n.d. not determined because of signal overlap or close proximity with the nitro group-bearing carbons. | ||||||
| 15d/20d | Ph | 3,5-(NO2)2Ph | 147.66 | 50.40 (5.87) | 157.68 | 55.55 (6.31) |
| 15e/20e | 2,4-(NO2)2Ph | 148.32 | 48.98 (6.03) | 157.60 | 54.19 (6.48) | |
| 16c/21c | 4-NO2Ph | 4-NO2Ph | 148.06 | 51.12 (5.74) | 156.75 | 56.41 (6.10) |
| 16d/21d | 3,5-(NO2)2Ph | n.d. | 50.47 (5.94) | 157.08 | 55.62 (6.32) | |
| 16e/21e | 2,4-(NO2)2Ph | 148.10 | 49.13 (6.09) | 156.91 | 54.26 (6.47) | |
| 17a/22a | 2,4-(NO2)2Ph | H | 147.14 | 34.67 (3.98) | 155.86 | 40.09 (4.39) |
| 17b/22b | Ph | n.d. | 52.13 (5.57) | 156.24 | 57.52 (5.90) | |
| 17c/22c | 4-NO2Ph | n.d. | 51.21 (5.79) | 156.73 | 56.47 (6.12) | |
| 17d/22d | 3,5-(NO2)2Ph | 147.79 | 50.59 (6.00) | 157.07 | 55.70 (6.33) | |
| 17e/22e | 2,4-(NO2)2Ph | n.d. | 49.39 (6.14) | 156.92 | 54.36 (6.49) | |
| 18a/23a | 3,5-(NO2)2Ph | H | 145.62 | 34.12 (3.94) | 154.74 | 39.78 (4.35) |
| 18b/23b | Ph | 146.86 | 52.09 (5.57) | 156.18 | 57.50 (5.87) | |
| 18c/23c | 4-NO2Ph | 148.01 | 50.34 (5.79) | 156.72 | 56.50 (6.10) | |
| 18d/23d | 3,5-(NO2)2Ph | 147.54 | 50.52 (5.99) | 155.68 | 55.71 (6.32) | |
| 18e/23e | 2,4-(NO2)2Ph | 148.00 | 49.29 (6.12) | 156.82 | 54.34 (6.47) | |
| 19d/24d | 4-MeOPh | 3,5-(NO2)2Ph | 147.74 | 50.38 (5.87) | 157.84 | 55.52 (6.31) |
| 19e/24e | 2,4-(NO2)2Ph | 148.38 | 48.90 (6.02) | 157.70 | 54.15 (6.47) | |
The synthetic procedure for 1-alkyl-5-(alkylsulfanyl)-1H-tetrazoles (33–36) and 2-alkyl-5-(alkylsulfanyl)-2H-tetrazoles (37–40) is shown in Scheme 2. Unlike selenium analogs, 5-(alkylsulfanyl)-1H-tetrazoles (29–32) were isolated in moderate yields (50–68%). Alkylation of the tetrazoles (29–32) was performed in THF or DMF in the presence of KOH or under phase-transfer catalysis conditions (compounds 36a, 36b, 40a, and 40b). 1-Alkyl-5-(alkylsulfanyl)-1H-tetrazoles (33–36) and their respective 2-alkyl isomers (37–40) were separated by column chromatography and were obtained in ratios ranging from 1 : 1.6 to 1 : 4. The identification of the 1,5- and 2,5-isomers was performed using the 1H and 13C NMR chemical shifts of the tetrazole carbon and the methylene group on the tetrazole nitrogen.31,32 These results followed the same rules as in the above-mentioned selenium derivatives: the signals of 2-alkyl-5-(alkylsulfanyl)-2H-tetrazoles were observed downfield compared to the 1-isomers (Table 2), and the 2-isomers had lower retention (higher values of Rf) on silica compared to the 1-isomers. The regioisomeric identities were confirmed by 1D NOESY experiments of compounds 33d and 37d, which showed correlations between methylene hydrogens in 1,5-regioisomer 33d only (see ESI†).
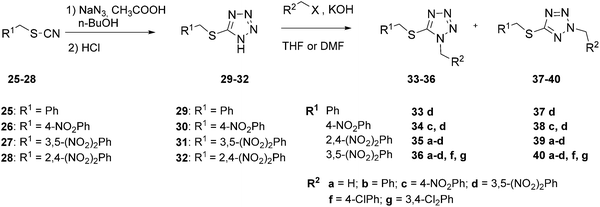 | ||
| Scheme 2 Synthesis of 1-alkyl and 2-alkyl-5-(alkylsulfanyl)-2H-tetrazoles. | ||
| 1-/2-Isomers | R1-CH2S | R2-CH2N | 1-Isomer | 2-Isomer | ||
|---|---|---|---|---|---|---|
| Ctetr | R2-CH2N1 | Ctetr | R2-CH2N2 | |||
| 33d/37d | Ph | 3,5-(NO2)2Ph | 154.65 | 49.80 (5.89) | 165.02 | 55.61 (6.27) |
| 34c/38c | 4-NO2Ph | 4-NO2Ph | 154.10 | 50.54 (5.74) | 164.06 | 56.45 (6.06) |
| 34d/38d | 3,5-(NO2)2Ph | 154.31 | 49.92 (5.95) | 164.37 | 55.69 (6.27) | |
| 35a/39a | 2,4-(NO2)2Ph | H | 153.77 | 34.77 (3.94) | 162.96 | 40.14 (4.33) |
| 35b/39b | Ph | 153.72 | 51.51 (5.53) | 163.35 | 57.57 (5.83) | |
| 35c/39c | 4-NO2Ph | 154.15 | 50.61 (5.75) | 163.83 | 56.53 (6.05) | |
| 35d/39d | 3,5-(NO2)2Ph | 154.38 | 49.94 (5.96) | 163.28 | 54.90 (6.26) | |
| 36a/40a | 3,5-(NO2)2Ph | H | 152.33 | 35.37 (3.92) | 162.02 | 39.82 (4.30) |
| 36b/40b | Ph | 153.06 | 50.51 (5.54) | 163.34 | 57.54 (5.83) | |
| 36c/40c | 4-NO2Ph | 153.91 | 50.61 (5.77) | 163.83 | 56.51 (6.05) | |
| 36d/40d | 3,5-(NO2)2Ph | 153.30 | 49.11 (5.97) | 163.25 | 54.92 (6.27) | |
| 36f/40f | 4-ClPh | 153.58 | 50.78 (5.57) | 163.52 | 56.74 (5.85) | |
| 36g/40g | 3,4-Cl2Ph | 152.15 | 50.00 (5.35) | 163.70 | 56.13 (5.89) | |
In vitro antimycobacterial activity
In vitro antimycobacterial activities of the synthesized compounds were evaluated against M. tb CNCTC My 331/88 and non-tuberculous mycobacteria – M. avium CNCTC My 330/88, M. kansasii CNCTC My 235/80 and M. kansasii 6509/96. All strains were obtained from the Czech National Collection of Type Cultures (CNCTC), with the exception of M. kansasii 6509/96, which was a clinical isolate. The activities of the compounds were determined in the Sula semisynthetic medium. Minimum inhibitory concentrations (MICs), i.e., the lowest concentrations that inhibit the visible growth of mycobacteria, were determined after incubation at 37 °C for 7, 14, and 21 days for both strains of M. kansasii and after 14 and 21 days for M. tb and M. avium. The values of MICs are expressed in μM and are presented in Tables 3 and 4. Isoniazid (INH) was used as a prototype drug.| R1 | R2 | M. tb My 331/88 | M. avium My 330/88 | M. kansasii My 235/80 | M. kansasii 6509/96 | ||
|---|---|---|---|---|---|---|---|
| 14/21 days | 7/14/21 days | ||||||
| a n.d. not determined. | |||||||
| 1,5-Regioisomers | 15d | Ph | 3,5-(NO2)2Ph | 8/8 | 32/32 | 8/16/32 | 16/32/32 |
| 15e | 2,4-(NO2)2Ph | 1/2 | 16/32 | 32/62/62 | 16/62/125 | ||
| 16c | 4-NO2Ph | 4-NO2Ph | 62/125 | 250/250 | 62/125/125 | 32/62/62 | |
| 16d | 3,5-(NO2)2Ph | 8/8 | 250/250 | 16/32/32 | 32/32/62 | ||
| 16e | 2,4-(NO2)2Ph | 2/4 | 16/32 | 32/62/62 | 32/62/62 | ||
| 17a | 2,4-(NO2)2Ph | H | 32/32 | 250/250 | 62/125/125 | 62/125/250 | |
| 17b | Ph | 8/16 | 250/250 | 16/32/125 | 16/62/125 | ||
| 17c | 4-NO2Ph | 16/16 | 62/62 | 16/32/32 | 32/62/62 | ||
| 17d | 3,5-(NO2)2Ph | 16/16 | 250/250 | 16/32/62 | 32/62/62 | ||
| 17e | 2,4-(NO2)2Ph | 8/16 | 32/32 | 32/62/62 | 32/62/62 | ||
| 18a | 3,5-(NO2)2Ph | H | 8/8 | 500/500 | 8/32/32 | 16/32/32 | |
| 18b | Ph | 1/2 | 32/32 | 2/4/4 | 4/8/8 | ||
| 18c | 4-NO2Ph | 2/4 | 125/125 | 4/16/32 | 4/8/16 | ||
| 18d | 3,5-(NO2)2Ph | 4/8 | 125/125 | 16/62/62 | 16/62/62 | ||
| 18e | 2,4-(NO2)2Ph | 4/8 | 125/125 | 8/32/62 | 4/16/32 | ||
| 19d | 4-CH3OPh | 3,5-(NO2)2Ph | 16/16 | 125/125 | 16/32/62 | 16/32/62 | |
| 19e | 2,4-(NO2)2Ph | 4/8 | 125/125 | 16/32/62 | 4/16/32 | ||
| 2,5-Regioisomers | 20d | Ph | 3,5-(NO2)2Ph | 8/8 | 16/16 | 8/16/32 | 16/32/32 |
| 20e | 2,4-(NO2)2Ph | 1/1 | 4/8 | 8/16/16 | 8/16/32 | ||
| 21c | 4-NO2Ph | 4-NO2Ph | 62/62 | 250/250 | 32/125/125 | 62/125/125 | |
| 21d | 3,5-(NO2)2Ph | 32/32 | 250/250 | 32/62/62 | 32/62/62 | ||
| 21e | 2,4-(NO2)2Ph | 2/2 | 8/16 | 1/2/2 | 2/4/8 | ||
| 22a | 2,4-(NO2)2Ph | H | 16/16 | 62/62 | 32/125/125 | 16/62/125 | |
| 22b | Ph | 16/16 | 62/62 | 16/16/32 | 32/32/62 | ||
| 22c | 4-NO2Ph | 32/32 | 125/125 | 16/32/32 | 32/62/62 | ||
| 22d | 3,5-(NO2)2Ph | 16/16 | 250/250 | 8/16/32 | 16/32/62 | ||
| 22e | 2,4-(NO2)2Ph | 2/4 | 16/32 | 16/32/62 | 32/62/62 | ||
| 23a | 3,5-(NO2)2Ph | H | 1/2 | 250/250 | 2/4/8 | 2/4/4 | |
| 23b | Ph | 1/1 | 16/32 | 1/1/1 | 1/1/2 | ||
| 23c | 4-NO2Ph | 1/1 | 125/125 | 1/2/4 | 2/4/4 | ||
| 23d | 3,5-(NO2)2Ph | 1/1 | 125/125 | 2/4/8 | 2/4/8 | ||
| 23e | 2,4-(NO2)2Ph | 2/2 | 125/125 | 4/8/16 | 2/8/16 | ||
| 24d | 4-CH3OPh | 3,5-(NO2)2Ph | 4/8 | 16/32 | 8/16/16 | 16/32/32 | |
| 24e | 2,4-(NO2)2Ph | n.d. | n.d. | n.d. | n.d. | ||
| INH | 0.5/1 | 250/250 | 250/250/250 | 4/4/4 | |||
| R1 | R2 | M. tb My 331/88 | M. avium My 330/88 | M. kansasii My 235/80 | M. kansasii 6509/96 | ||
|---|---|---|---|---|---|---|---|
| 14/21 days | 7/14/21 days | ||||||
| a n.d. not determined. | |||||||
| 1,5-Regioisomers | 33d | Ph | 3,5-(NO2)2Ph | 8/8 | 125/125 | 32/62/62 | 16/32/32 |
| 34c | 4-NO2Ph | 4-NO2Ph | 500/500 | 250/250 | 250/250/250 | 250/250/250 | |
| 34d | 3,5-(NO2)2Ph | 8/8 | 250/250 | 4/8/16 | 16/16/32 | ||
| 35a | 2,4-(NO2)2Ph | H | 16/16 | 125/500 | 125/500/500 | 125/500/500 | |
| 35b | Ph | 8/8 | 250/250 | 8/32/62 | 16/32/62 | ||
| 35c | 4-NO2Ph | 4/4 | 62/62 | 32/62/62 | 32/62/62 | ||
| 35d | 3,5-(NO2)2Ph | 4/4 | 16/32 | 32/62/62 | 16/32/62 | ||
| 36a | 3,5-(NO2)2Ph | H | 4/8 | 500/1000 | 4/16/32 | 16/32/32 | |
| 36b | Ph | 1/2 | 250/250 | 2/8/8 | 2/4/4 | ||
| 36c | 4-NO2Ph | 2/4 | 250/250 | 1/4/4 | 2/8/16 | ||
| 36d | 3,5-(NO2)2Ph | 4/8 | 250/250 | 4/8/8 | 8/16/32 | ||
| 36f | 4-ClPh | 2/4 | 125/125 | 2/2/4 | 4/8/8 | ||
| 36g | 3,4-Cl2Ph | 2/2 | 125/125 | 2/4/4 | 2/4/8 | ||
| 2,5-Regioisomers | 37d | Ph | 3,5-(NO2)2Ph | 16/16 | 125/125 | 16/32/32 | 16/32/32 |
| 38c | 4-NO2Ph | 4-NO2Ph | 250/250 | 250/250 | 250/250/250 | 250/250/250 | |
| 38d | 3,5-(NO2)2Ph | 8/8 | 250/250 | 4/8/16 | 16/16/32 | ||
| 39a | 2,4-(NO2)2Ph | H | 4/8 | 125/125 | 32/125/125 | 32/125/125 | |
| 39b | Ph | 4/4 | 32/62 | 16/32/62 | 8/16/32 | ||
| 39c | 4-NO2Ph | n.d. | n.d. | n.d. | n.d. | ||
| 39d | 3,5-(NO2)2Ph | 4/4 | 250/250 | 4/16/32 | 8/16/16 | ||
| 40a | 3,5-(NO2)2Ph | H | 2/4 | 125/250 | 4/8/8 | 8/16/16 | |
| 40b | Ph | 1/1 | 62/125 | 8/16/16 | 4/4/8 | ||
| 40c | 4-NO2Ph | 1/2 | 250/250 | 2/2/4 | 2/8/8 | ||
| 40d | 3,5-(NO2)2Ph | 1/2 | 250/250 | 1/2/2 | 2/4/8 | ||
| 40f | 4-Cl | 1/2 | 16/16 | 4/8/16 | 16/16/32 | ||
| 40g | 3,4-Cl2Ph | 1/1 | 125/125 | 8/16/32 | 16/32/32 | ||
| INH | 0.5/1 | 250/250 | 250/250/250 | 4/4/4 | |||
The antimycobacterial activities of the most potent compounds in the series of 1- and 2-isomers of selanyltetrazoles (15–24) and sulfanyltetrazoles (33–40) were 1 μM against M. tb, which is equivalent to the first-line anti-TB drug isoniazid (INH), and 1–2 μM against both INH-resistant and INH-susceptible M. kansasii. These results support our previous observations that 3,5-dinitrobenzylsulfanyl/selanyl derivatives had significantly higher activities than 2,4-dinitrobenzylsulfanyl/selanyl derivatives and both nitro groups are necessary for the high antimycobacterial efficiency of the studied compounds. Hence, the most active substances were 1-alkyl-5-[(3,5-dinitrobenzyl)selanyl]-1H-tetrazoles (18a–e), 2-alkyl-5-[(3,5-dinitrobenzyl)selanyl]-2H-tetrazoles (23a–e), 1-alkyl-5-[(3,5-dinitrobenzyl)sulfanyl]-1H-tetrazoles (36a–d, 36f, 36g) and 2-alkyl-5-[(3,5-dinitrobenzyl)sulfanyl]-2H-tetrazoles (40a–d, 40f, 40g). Considering the position of the alkyl substituent, the 2-isomer series (23 and 40) exhibited higher activity than the 1-isomer series (18 and 36). Interestingly, the antitubercular activities of the 2-isomers 23a–e, 40a–d, 40f and 40g reached MIC values of 1–2 μM regardless of the substituent on the tetrazole cycle. Conversely, the activities of the corresponding 1-isomers 18a–e, 36a–d, 36f and 36g, with MIC values ranging from 1 to 8 μM, appeared to be more influenced by the type of substituent on the tetrazole than their corresponding 2-isomers. The combination of a 3,5-dinitrobenzyl substituent on sulfur/selenium and a dinitrobenzyl substituent in position 1 of tetrazole (18d, 18e, and 36d) decreased antimycobacterial activity compared to substances with a 3,5-dinitrobenzyl substituent on sulfur/selenium and a 1-benzyl (18b, 36b) or 1-(4-nitrobenzyl) substituent (18c, 36c) on tetrazole. The MIC values of benzylselanyl/3,5-dinitrobenzyl and 3,5-dinitrobenzylselanyl/benzyl reverse analog pairs 15d/18b and 20d/23b and their respective sulfanyl reverse analog pairs 33d/36b and 37d/40b showed that the position of the 3,5-dinitrobenzyl substituent on sulfur/selenium is beneficial, while the 3,5-dinitrobenzyl substituted tetrazole moiety was generally unfavorable for antimycobacterial activity. Isomers 15e and 20e bearing a 2,4-dinitrobenzyl moiety on tetrazole were exceptions and exhibited surprisingly high antimycobacterial activity. However, this observation is likely connected with the high non-selective toxicity of 2,4-dinitrobenzyl derivatives (see below).
Nitro group-containing anti-TB agents display various mechanisms of antimycobacterial action such as inhibition of DprE1 (dinitrobenzamides, benzothiazinones),11 inhibition of mycolic acid biosynthesis or NO poisoning of cytochrome c oxidase (PA-824, delamanid).33 Although the most potent compounds 23a–e, 40a–d, 40f and 40g contain 3,5-dinitrophenyl fragments, such as the DprE1 inhibiting dinitrobenzamides, their actual mechanism of action remains to be elucidated.
In vitro antibacterial and antifungal activity
The selectivities of the antimycobacterial effect of compounds 18b, 23b, 36b, 40b and 40d, which exhibited promising antimycobacterial activity, were evaluated by determining the MIC values against 8 bacterial and 8 fungal strains. All compounds showed no antibacterial and no antifungal activity (Tables S1 and S2, see ESI†).In vitro cell proliferation/viability assays
To further probe the selectivity of the studied compounds, their effects on the viability of mammalian cells were evaluated. We were also interested in how the type and position of the substituents on tetrazole or on the sulfur/selenium atom and the presence of either sulfur or selenium in position 5 of tetrazole would influence the overall toxicity of the studied compounds. Therefore, the effects of five 1,5- and 2,5-regioisomeric pairs of the selenium derivatives, 15d/20d, 17b/22b, 18b/23b, 18c/23c and 18d/23d, five pairs of the sulfur regioisomers, 33d/37d, 36b/40b, 36c/40c, 36f/40f and 36g/40g, and compounds 39b and 40d on the viability of the Chinese hamster ovary (CHO–K1) cell line were evaluated (Table 5).| 1-Isomer | IC50 | 2-Isomer | IC50 |
|---|---|---|---|
| a n.d. not determined. | |||
| 15d | 85 ± 21 | 20d | 118 ± 19 |
| 17b | 8.5 ± 0.2 | 22b | 22 ± 2 |
| 18b | 71 ± 34 | 23b | 136 ± 6 |
| 18c | 27 ± 14 | 23c | 205 ± 29 |
| 18d | 6.5 ± 0.2 | 23d | 40 ± 9 |
| 33d | 115 ± 18 | 37d | 148 ± 11 |
| 35b | n.d. | 39b | 21 ± 1.4 |
| 36b | 59 ± 11 | 40b | 41 ± 6 |
| 36c | 163 ± 40 | 40c | 243 ± 6 |
| 36d | n.d. | 40d | 133 ± 5 |
| 36f | 94 ± 16 | 40f | 301 ± 9 |
| 36g | 140 ± 9 | 40g | 150 ± 34 |
The resulting IC50 values indicated that there is no significant difference in the toxicity between sulfur or selenium derivatives. The 2,4-dinitro derivatives (17b, 22b and 39b) were highly toxic regardless of their substitution (IC50 values of 8.5–22 μM). The toxicities of the 1-isomers were either similar to or higher than the 2-isomers; this is observed in the IC50 values of the isomeric pairs 18b/23b, 18c/23c, 18d/23d, 36c/40c and 36f/40f. The most toxic compound, 18d, with an IC50 value of 6.5 μM has four nitro groups in its structure; however, there is no clear correlation between toxicity and the number of nitro groups present. Interestingly, introduction of a chlorine atom to the molecule decreased its toxicity (as in 36b and 36f or 40b and 40f); however, this effect did not show a clear dependence on the number of chlorine atoms, as observed in compounds 36g and 40g.
Ames fluctuation test
The mutagenic activity of regioisomeric pairs 18b/23b, 36b/40b, 36c/40c and 36f/40f was detected using the 96-well micro-plate version of the Salmonella typhimurium Ames Test. At 50 μM we found highly variable potencies of the tested compounds to induce reverse mutation on the S. typhimurium strain TA98 and no mutagenicity on the strain TA100, with no apparent structure–mutagenicity relationships. Importantly, compound 36b did not induce any mutation in both strains. This indicates that the 3,5-dinitrobenzyl moiety is not generally connected with frame shift or base-exchange mutagenicity (see ESI†).Conclusions
In this study, a series of nitro group-containing regioisomeric 1-alkyl- and 2-alkyl-5-(alkylsulfanyl)-2H-tetrazoles and their selenium bioisosteres were prepared and characterized. All 1-alkyl and 2-alkyl regioisomers were isolated and unambiguously identified by the 1H and 13C NMR chemical shifts of the methylene group adjacent to the tetrazole nitrogen and the 13C NMR chemical shift of the tetrazole carbon. The regioisomeric identities were confirmed by NOESY experiments.Antimycobacterial evaluation indicated that 1-alkyl- (36a–d, 36f, 36g) and 2-alkyl-5-[(3,5-dinitrobenzyl)sulfanyl]-2H-tetrazoles (40a–d, 40f, 40g) and their selenium analogs (18a–e, 23a–e) exhibited promising in vitro antimycobacterial activity against M. tb CNCTC My 331/88, with MIC values as low as 1 μM. These derivatives also showed high activities against non-tuberculous M. kansasii 6509/96 and INH-resistant M. kansasii CNCTC My 235/80, with MIC values similar to or slightly lower than that of M. tb. Furthermore, the antimycobacterial effects of these compounds were found to be highly specific, because they showed no antibacterial or antifungal activity and low cytotoxicity in a mammalian cell line. Interestingly, no differences in these biological properties between sulfur and selenium bioisosteres were found. We also found that the 3,5-dinitrobenzyl moiety per se is not the carrier of mutagenicity.
The structure–activity relationship study showed that the position of the 3,5-dinitrobenzyl substituent on the sulfur/selenium atom in position 5 of tetrazole is beneficial, as the reverse analogs, i.e., 1- or 2-(3,5-dinitrobenzyl)tetrazole derivatives, exhibited significantly lower antimycobacterial activities. Derivatives bearing a 2,4-dinitrobenzyl moiety generally exhibited lower antimycobacterial activities and higher in vitro cytotoxicity compared to the 3,5-dinitrobenzyl derivatives. Considering the position of the alkyl substituent on tetrazole, 2-alkyl-5-[(3,5-dinitrobenzyl)sulfanyl]-2H-tetrazoles and their selenium analogs showed higher antimycobacterial activity against M. tb and lower cytotoxicity compared to the 1-alkyl isomers. Consequently, 2-alkyl-5-[(3,5-dinitrobenzyl)sulfanyl]-2H-tetrazoles (40) are new lead antimycobacterial compounds because they are superior to 1-alkyl derivatives in their antimycobacterial effect and exhibit lower cytotoxicity. Moreover, the antimycobacterial activity of the 1-alkyl isomers was more influenced by the type of alkyl substituent than were the 2-alkyl isomers. Thus, variation of the 2-alkyl substituent may further optimize the ADME properties and toxicity of these compounds while maintaining the antimycobacterial efficiency.
Acknowledgements
This work was supported by the Czech Science Foundation project 14-08423S and MH CZ – DRO (UHHK, 00179906). G.K. thanks Charles University in Prague (SVV 260 062).Notes and references
- World Health Organization, Global tuberculosis report, 2012, http://www.who.int/tb Search PubMed.
- A. Zumla, P. Nahid and S. T. Cole, Nat. Rev. Drug Discovery, 2013, 12, 388–404 CrossRef CAS PubMed.
- Beena and D. S. Rawat, Med. Res. Rev., 2013, 33, 693–764 CrossRef CAS PubMed.
- M. Yan and S. T. Ma, ChemMedChem, 2012, 7, 2063–2075 CrossRef CAS PubMed.
- C. Lienhardt, M. Raviglione, M. Spigelman, R. Hafner, E. Jaramillo, M. Hoelscher, A. Zumla and J. Gheuens, J. Infect. Dis., 2012, 205, S241–S249 CrossRef PubMed.
- A. J. Lenaerts, V. Gruppo, K. S. Marietta, C. M. Johnson, D. K. Driscoll, N. M. Tompkins, J. D. Rose, R. C. Reynolds and I. M. Orme, Antimicrob. Agents Chemother., 2005, 49, 2294–2301 CrossRef CAS PubMed.
- M. Matsumoto, H. Hashizume, T. Tomishige, M. Kawasaki, H. Tsubouchi, H. Sasaki, Y. Shimokawa and M. Komatsu, PLoS Med., 2006, 3, 2131–2144 CAS.
- M. T. Gler, V. Skripconoka, E. Sanchez-Garavito, H. P. Xiao, J. L. Cabrera-Rivero, D. E. Vargas-Vasquez, M. Q. Gao, M. Awad, S. K. Park, T. S. Shim, G. Y. Suh, M. Danilovits, H. Ogata, A. Kurve, J. Chang, K. Suzuki, T. Tupasi, W. J. Koh, B. Seaworth, L. J. Geiter and C. D. Wells, N. Engl. J. Med., 2012, 366, 2151–2160 CrossRef CAS PubMed.
- T. Christophe, M. Jackson, H. K. Jeon, D. Fenistein, M. Contreras-Dominguez, J. Kim, A. Genovesio, J. P. Carralot, F. Ewann, E. H. Kim, S. Y. Lee, S. Kang, M. J. Seo, E. J. Park, H. Skovierova, H. Pham, G. Riccardi, J. Y. Nam, L. Marsollier, M. Kempf, M. L. Joly-Guillou, T. Oh, W. K. Shin, Z. No, U. Nehrbass, R. Brosch, S. T. Cole and P. Brodin, PLoS Pathog., 2009, 5, e1000645 Search PubMed.
- V. Makarov, G. Manina, K. Mikusova, U. Mollmann, O. Ryabova, B. Saint-Joanis, N. Dhar, M. R. Pasca, S. Buroni, A. P. Lucarelli, A. Milano, E. De Rossi, M. Belanova, A. Bobovska, P. Dianiskova, J. Kordulakova, C. Sala, E. Fullam, P. Schneider, J. D. McKinney, P. Brodin, T. Christophe, S. Waddell, P. Butcher, J. Albrethsen, I. Rosenkrands, R. Brosch, V. Nandi, S. Bharath, S. Gaonkar, R. K. Shandil, V. Balasubramanian, T. Balganesh, S. Tyagi, J. Grosset, G. Riccardi and S. T. Cole, Science, 2009, 324, 801–804 CrossRef CAS PubMed.
- C. Trefzer, H. Skovierova, S. Buroni, A. Bobovska, S. Nenci, E. Molteni, F. Pojer, M. R. Pasca, V. Makarov, S. T. Cole, G. Riccardi, K. Mikusova and K. Johnsson, J. Am. Chem. Soc., 2012, 134, 912–915 CrossRef CAS PubMed.
- V. Makarov, B. Lechartier, M. Zhang, J. Neres, A. M. van der Sar, S. A. Raadsen, R. C. Hartkoorn, O. B. Ryabova, A. Vocat, L. A. Decosterd, N. Widmer, T. Buclin, W. Bitter, K. Andries, F. Pojer, P. J. Dyson and S. T. Cole, EMBO Mol. Med., 2014, 6, 372–383 CrossRef CAS PubMed.
- Z. Kazimierczuk, M. Andrzejewska, J. Kaustova and V. Klimesova, Eur. J. Med. Chem., 2005, 40, 203–208 CrossRef CAS PubMed.
- V. Klimesova, J. Koci, M. Pour, J. Stachel, K. Waisser and J. Kaustova, Eur. J. Med. Chem., 2002, 37, 409–418 CrossRef CAS.
- J. Koci, V. Klimesova, K. Waisser, J. Kaustova, H. M. Dahse and U. Mollmann, Bioorg. Med. Chem. Lett., 2002, 12, 3275–3278 CrossRef CAS.
- V. Klimesova, J. Koci, K. Palat, J. Stolarikova, H. M. Dahse and U. Mollmann, Med. Chem., 2012, 8, 281–292 CrossRef CAS.
- G. Karabanovich, J. Roh, T. Smutný, J. Němeček, P. Vicherek, J. Stolaříková, M. Vejsová, I. Dufková, K. Vávrová, P. Pávek, V. Klimešová and A. Hrabálek, Eur. J. Med. Chem., 2014, 82, 324–340 CrossRef CAS PubMed.
- G. I. Koldobskii and R. B. Kharbash, Russ. J. Org. Chem., 2003, 39, 453–470 CrossRef CAS.
- G. I. Koldobskii, Russ. J. Org. Chem., 2006, 42, 469–486 CrossRef CAS.
- J. Roh, K. Vavrova and A. Hrabalek, Eur. J. Org. Chem., 2012, 6101–6118 CrossRef CAS.
- G. Ortar, M. G. Cascio, A. S. Moriello, M. Camalli, E. Morera, M. Nalli and V. Di Marzo, Eur. J. Med. Chem., 2008, 43, 62–72 CrossRef CAS PubMed.
- M. Sabbah, F. Fontaine, L. Grand, M. Boukraa, M. L. Efrit, A. Doutheau, L. Soulere and Y. Queneau, Bioorg. Med. Chem., 2012, 20, 4727–4736 CrossRef CAS PubMed.
- J. Garfunkle, C. Ezzili, T. J. Rayl, D. G. Hochstatter, I. Hwang and D. L. Boger, J. Med. Chem., 2008, 51, 4392–4403 CrossRef CAS PubMed.
- G. Roman, M. N. Rahman, D. Vukomanovic, Z. C. Jia, K. Nakatsu and W. A. Szarek, Chem. Biol. Drug Des., 2010, 75, 68–90 CAS.
- M. Kume, T. Kubota, Y. Kimura, H. Nakashimizu and K. Motokawa, J. Pharm. Soc. Jpn., 1992, 112, 622–637 CAS.
- U. Muh, M. Schuster, R. Heim, A. Singh, E. R. Olson and E. P. Greenberg, Antimicrob. Agents Chemother., 2006, 50, 3674–3679 CrossRef CAS PubMed.
- A. D. White, M. W. Creswell, A. W. Chucholowski, C. J. Blankley, M. W. Wilson, R. F. Bousley, A. D. Essenburg, K. L. Hamelehle, B. R. Krause, R. L. Stanfield, M. A. Dominick and M. Neub, J. Med. Chem., 1996, 39, 4382–4395 CrossRef CAS PubMed.
- H. Ozkan, S. Yavuz, A. Disli, Y. Yildirir and L. Turker, Heteroat. Chem., 2007, 18, 255–258 CrossRef CAS.
- A. Disli and M. Salman, Russ. J. Org. Chem., 2009, 45, 151–153 CrossRef CAS.
- G. Karabanovich, J. Roh, Z. Padelkova, Z. Novak, K. Vavrova and A. Hrabalek, Tetrahedron, 2013, 69, 8798–8808 CrossRef CAS PubMed.
- R. E. Trifonov and V. A. Ostrovskii, Russ. J. Org. Chem., 2006, 42, 1585–1605 CrossRef CAS.
- N. N. Sveshnikov and J. H. Nelson, Magn. Reson. Chem., 1997, 35, 209–212 CrossRef CAS.
- U. Manjunatha, H. I. Boshoff and C. E. Barry, Commun. Integr. Biol., 2009, 2, 215–218 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of chemical synthesis and characterization of all the reported compounds. Details of in vitro antimycobacterial, antibacterial, antifungal, cell proliferation/viability assays and AMES fluctuation test. Tables S1 and S2. See DOI: 10.1039/c4md00301b |
| This journal is © The Royal Society of Chemistry 2015 |