Copper and antimony isotopic analysis via multi-collector ICP-mass spectrometry for provenancing ancient glass†
Lara
Lobo
*a,
Patrick
Degryse
b,
Andrew
Shortland
c,
Katherine
Eremin
d and
Frank
Vanhaecke
a
aDepartment of Analytical Chemistry, Ghent University, Krijgslaan 281-S12, 9000 Ghent, Belgium. E-mail: Lara.LoboRevilla@UGent.be
bDepartment of Earth and Environmental Sciences, Katholieke Universiteit Leuven, Celestijnenlaan 200 E – box 2408, 3001 Heverlee, Belgium
cDepartment of Engineering and Applied Science, Cranfield University, Shrivenham, Swindon SN6 8LA, UK
dHarvard Art Museums, 32 Quincy Street, Cambridge, MA 02138, USA
First published on 4th November 2013
Abstract
Variations in the isotopic composition of Cu and Sb as determined using multi-collector ICP-mass spectrometry (MC-ICPMS) have been investigated as a proxy for provenancing ancient glass. Cu and Sb were added during the manufacturing of ancient (pre-Roman and Roman) glass to obtain colour and opacity. In previous work, the analytical methodology for sample digestion and isolation of Sb preceding isotopic analysis via multi-collector ICP-MS was developed. Although applications of Cu isotopic analysis can be found in the literature, this approach has not been used for provenancing glass raw materials yet. Therefore, the protocols for digestion and Cu isolation were optimized and validated, relying on the use of both an in-house multi-elemental standard and NIST SRM 610 glass reference material. The methods for Sb and Cu isotopic analysis were subsequently applied to a series of late Bronze Age Mesopotamian–Egyptian to Hellenistic–Roman glasses. Results obtained show that the isotopic composition of Cu, expressed as δ65Cu, varies from −1.9 to −0.2‰, thus covering a range of approximately 2‰. Unfortunately, the use of Cu isotope ratios to characterize raw materials used in glass manufacturing is complicated by the fact that Cu ores from within a single deposit can exhibit a similar range in δ65Cu values, certainly for co-existing Cu sulfides and oxides. Sb in stibnite ore, on the other hand, only shows a variance in isotopic composition of ∼10 ε units (or 0.1‰), but Sb isotopic analysis offers more potential to pinpoint the location of an antimony source used in antiquity.
Introduction
The first glass objects (recovered from excavations) are reported to stem from the third millennium BC in Mesopotamia and Egypt. Glass was initially a prestigious material with assumed magical properties, mostly owned by royalty and their court.1 During the Hellenistic and Roman era, glass was produced in great volume and was no longer uniquely a luxury good, but used in every-day life.2 Glass was made using three main components:3 (i) a silica source in the form of sand or pure quartz, (ii) a stabilizer, mostly lime, added with the silica source or as a separate constituent, and (iii) a flux, in the form of plant ashes or mineral soda, to lower the silica melting point.Also colourants and decolourants were used in the glass production process. Co and Cu were used from the late Bronze Age in Egypt and Mesopotamia to produce blue colours. Co was added as cobaltiferous alum, an iron-rich alum containing cobalt4 (not excluding other possible sources), whereas the presence of Cu in glass has been assigned to the addition of Cu in the metallic form or as a Cu ore.1 Apart from turquoise-blue, also the more complex red colour5 of glass was obtained by the addition of Cu. Turquoise-blue (or green when using a higher temperature6) was obtained using oxidising conditions in the furnace, whereas red was achieved under strong reducing conditions. Sb was widely used as an opacifier in glass (Sb content >1%) from the late Bronze Age onwards, until Sn-based opacifiers were introduced around the 4th century AD.7 Calcium antimonate (white) and lead antimonate (yellow) are also commonly found,8 while Sb was also added to blue glasses to achieve opaque turquoise.1 Additionally, Sb is known as a decolourant (∼0.5% Sb) from the Greco-Roman period onward.9
Primary production locations of late Bronze Age glass have been clearly identified in Egypt, while their presence has also been suggested in Mesopotamia (and less likely in Greece).10 Primary glass furnaces during the Hellenistic and Roman period are known for Syro-Palestine and Egypt, but also primary production in the western Roman empire has been suggested.11 The origin of the materials used for glass production and the trade of glass in antiquity are currently very actively studied topics of research. Major, minor and trace elements have been used to provenance the raw materials of ancient glass making, as some elements were proved to be directly related to mineral raw materials in the silica source, colorants or flux.12,13 Isotopic analysis of Sr and Nd has also allowed to distinguish different raw material sources, and the use of O isotopic analysis in this context has been reported in the literature as well.14 Recently, we have reported on the use of Sb isotopic analysis as a novel tool for provenancing Sb-containing colourants/decolourants.15,16
In the present work, we have combined isotopic analysis of Sb with that of Cu and applied it to late Bronze Age Mesopotamian–Egyptian and Hellenistic–Roman glasses from the eastern part of the Roman empire. For Cu, different isolation procedures can be found in the literature,17–19 however, to the best of the authors' knowledge, Cu isotopic analysis has not been applied to glass materials yet. This makes evaluation and validation of the sample pretreatment preceeding Cu isotopic analysis necessary. Two different separation methods were selected for this purpose.18,19 Further tuning of the selected protocol was carried out, and the Cu–Sb isotopic composition of the ancient glass was evaluated.
Experimental
Instrumentation and reagents
Further purification of trace analysis purity level 14 M HNO3 and 12 M HCl (Chem-Lab, Belgium) was carried out by sub-boiling distillation in PFA and quartz equipment, respectively. Ultrapure 28.9 M HF was acquired from Thermo Fisher Scientific (Belgium) and 13.4 M NH4OH from Fluka (Belgium). Both reagents were used as such in the sample pretreatment. Ultrapure 9.8 M H2O2 and L-ascorbic acid (≥99% purity) were purchased from Sigma Aldrich (Belgium). Ultrapure water (resistivity ≥18.2 MΩ cm) was obtained from a Milli-Q Element water purification system (Millipore, France). Sample digestion was carried out in Savillex® beakers, after their cleaning with both HNO3 and HCl of pro analysis purity level (ProLabo, Belgium) and subsequently repeated rinsing with Milli-Q water. All sample pretreatment was carried out in a class-10 clean lab.1 g l−1 stock solutions used to prepare an in-house standard (57 elements from Li to U) were purchased from SCP Science (France), Inorganic Ventures (The Netherlands) and Chem-Lab (Belgium). 1 ml of the Na stock solution, 150 μl of that of Al, 50 μl of those of the other 54 “matrix” elements and 10 μl of that of Cu were pipetted into a beaker and the mixture thus obtained evaporated until dryness. After reconstitution in (i) 0.01 M HCl − 0.02 M L(+)-ascorbic acid or (ii) 8 M HCl + 0.001% H2O2, this in-house standard was used for validation of two different Cu isolation procedures.
Elemental concentrations were measured using a quadrupole-based ICP-MS instrument (Thermo Scientific XSeries II). Ga and Ru were used as internal standards to correct for matrix effects and instrument instability for Cu and Sb, respectively. Te is not present in glass and so, no isobaric interference from 123Te will occur. 65Cu/63Cu and 123Sb/121Sb isotope ratio determination was accomplished using a Thermo Scientific Neptune multi-collector ICP-MS instrument (MC-ICP-MS). Table 1 shows the instrumental settings and cup configurations used for the experiments. Samples were introduced into the ICP ion source using a 100 μl min−1 concentric nebulizer and a double spray chamber, consisting of a combination of a cyclonic sub-unit and a Scott-type one. The Neptune is equipped with 9 Faraday cups (8 of which mobile), connected to 1011 Ω amplifiers. Samples were run in a sample-standard bracketing sequence with a 500 μg l−1 Cu solution of NIST SRM 976 (NIST, USA) for external correction and 500 μg l−1 of admixed Zn for internal correction for mass bias. Similarly, Sb isotope ratios were corrected for mass bias using a standard solution of 200 μg l−1 Sb and 250 μg l−1 of In as an internal standard. All measured solutions were in a 0.42 M HNO3 matrix and the sample introduction system was rinsed with 0.42 M HNO3 in-between every two measurements. Prior to off-line mass bias correction, the data were processed on-line. This processing consisted of calculation of the ion beam intensities and isotope ratios and the removal of outliers based on a 2s-test.
| Instrumental settings Neptune | Cu | Sb |
|---|---|---|
| RF power (W) | 1200 | 1200 |
| Cool gas flow rate (l min−1) | 15 | 15 |
| Auxiliary gas flow rate (l min−1) | 0.60 | 0.60 |
| Nebulizer gas flow rate (l min−1) | 1.023 | 1.011 |
| Sampler cone | Ni: 1.1 mm | Ni: 1.1 mm |
| Skimmer | Ni, H-type: 0.8 mm | Ni, H-type: 0.8 mm |
| Sample uptake rate (ml min−1) | 0.10 | 0.10 |
| X-position (mm) | 3.740 | 3.720 |
| Y-position (mm) | −1.650 | −1.660 |
| Resolution | Medium | Low |
| Mode | Static | Static |
| Data acquisition parameters | Cu | Sb |
| Integration time (s) | 4 | 4 |
| Blocks | 9 | 7 |
| Cycles/block | 5 | 5 |
| Cup configuration | L3: 63Cu; L2: 64Zn: L1: 65Cu; C: 66Zn; H1: 67Zn; H2: 68Zn | L2: 115In; C: 113In, H2: 121Sb; H3: 123Sb |
Samples
The NIST SRM 610 glass reference material (NIST, USA) was used for validation of the digestion and isolation procedures. This glass material has certified mass fractions of 444 ± 4 μg g−1 of Cu and 415.3 ± 3.7 μg g−1 of Sb. Table 2 shows the glasses analysed in the present work, originating from late Bronze Age Egypt (4) and Mesopotamia (2 from Nuzi, 6 from Nippur) and the eastern part of the Greco-Roman world (2 from Pichvnari and 5 from Sagalassos). As the archaeological samples are small, with a weight from 5 mg to 30 mg, only a single digestion was possible for most of the samples. Nevertheless, whenever possible, two sub-samples of each glass (indicated as a and b in Table 2) were used for the experiments. Cu and Sb concentrations determined by Q-ICP-MS have also been indicated. Most of the glass samples were free from Co, however, samples with a Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co ratio up to 7
Co ratio up to 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (e.g., B2496.10) were encountered as well.
2 (e.g., B2496.10) were encountered as well.
| Location | Sample | Period | Cu (%) | Sb (%) |
|---|---|---|---|---|
| Malkata (Egypt) | COP3: white | 14th century BC | Not determined | 0.39 |
| COP14: green | 0.98 | 0.20 | ||
| UPP37; turquoise | Not determined | 0.41 | ||
| UPP40; dark blue/white | 0.09 | 1.15 | ||
| Nuzi, (Mesopotamia) | 1930.82.50-1a; blue | 14th century BC | 1.36 | 0.05 |
| 1930.82.50-1b | ||||
| 1930.68.15; blue | 0.65 | 0.03 | ||
| Nippur, (Mesopotamia) | B2496/1(8); dark blue | 13th–14th century BC | 0.58 | 1.94 |
| B2496/3(5); turquoise | 1.27 | 1.90 | ||
| B2496/3(6)-1a; turquoise | ||||
| B2496/3(6)-1b | 1.34 | 1.92 | ||
| B2496.8; brown | 0.37 | 1.94 | ||
| B2496.10; blue/brown | 0.33 | 1.09 | ||
| B2496.5; dark blue | 0.89 | 0.17 | ||
| Pichvnari (Georgia) | Op30 (Pic30b); white | 3rd–5th century BC | 0.19 | 1.54 |
| Op31 (Pic31b); turquoise | 0.67 | 1.10 | ||
| Sagalassos (Turkey) | Turquoise-1 | 5th–6th century AD | 0.98 | 0.48 |
| Turquoise-2 | 1.56 | 0.99 | ||
| Black-2 | 0.05 | 1.44 | ||
| Green-1a | 1.72 | 0.64 | ||
| Green-1b | 1.77 | 0.58 | ||
| Green-yellow-1 | 0.76 | 1.06 |
Digestion
Sample digestion was realized using the two-step procedure described in a previous paper.16 First, a mixture of HF and HNO3 was used to dissolve the oxides at 110 °C for 48 h. Then, the digestion was continued at the same temperature and duration in aqua regia. Evaporation steps were carried out at 90 °C only, to avoid any loss of Sb by evaporation.20 Finally, the residue obtained was redissolved in 10 ml of 0.14 M HF. 5 ml of this digest was used for Sb isotopic analysis, the other 5 ml were evaporated and redissolved in 3 ml of 8 M HCl to perform Cu isotopic analysis. Quantitative recovery was obtained for both Sb and Cu from the NIST SRM 610 glass reference material.Mass bias correction
To correct for instrumental mass discrimination, the revised Russell's law21 was used for both Cu and Sb.Variations in the isotopic composition of Cu were finally expressed as δ65Cu values, calculated with respect to the NIST SRM 976 copper isotopic reference material (NIST, USA). On the other hand, Sb isotope ratio variations were expressed in ε123Sb units, calculated with respect to an in-house standard (SPC Science, F2-SB03010), since there is no isotopic reference material available for Sb. The internal precision achieved was 0.003–0.005% RSD for Cu and 0.002–0.006% RSD for Sb, respectively.
Results and discussion
Isolation procedure
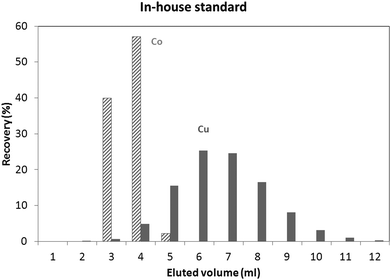
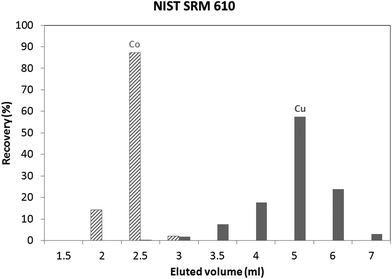
An in-house standard (the composition of which is described in the Experimental section) was initially used to evaluate the utility of both isolation procedures for glass samples. Results obtained using these protocols showed a Cu recovery of 78% and 100%, for the “Larner” and “Van Heghe” protocols, respectively. Therefore, the latter method was selected for further validation. Table 3 shows the recoveries obtained for all the elements present in the in-house standard after 1 ml sample loading. It can be seen that the large majority of the elements are not eluted in the Cu fraction. Only Co is co-eluted with Cu, whereas In, Ta and Re are partially eluted in the Cu fraction. While the latter elements are not commonly present in glass, Co can be found in glasses together with Cu and so, further tuning of the separation process is required. Fig. 1 shows the elution profile obtained upon separate collection and analysis of individual 1 ml aliquots of the Cu fraction (in-house standard). It can be seen that Cu starts to elute after 3 ml, at the same time as Co. However, only ∼6% of the Cu (fraction 3 + 4) elutes with ∼97% of the Co. In other words, ∼94% of the Cu elutes almost free of Co. However, it is well known that there is on-column fractionation of Cu during anion exchange processes23 – a δ65Cu value of 0.73‰ was reported at 82% Cu recovery and a δ65Cu value of 0.15‰ at 98% Cu recovery for a standard solution with respect to NIST SRM 97617 such that quantitative recovery is required prior to isotope ratio determination. It has been reported that Co can be eluted from AG 1-X8 anion exchange resin with 4 M HCl,24,25 such that lower acid concentrations were tested in an effort to obtain a proper separation of Co and Cu. 3.5 M HCl was finally selected as the optimum mobile phase. The elution profile obtained under these conditions for the NIST SRM 610 glass reference material is shown in Fig. 2. It can be seen that smaller fractions (0.5 ml) were collected at the beginning of the elution profile in order to be able to evaluate the separation of the analytes in sufficient detail. Although Co and Cu are now eluted at different fractions, still a small amount of Co (∼2%) is co-eluted with Cu (∼2%). However, the separation achieved is sufficient to warrant reliable Cu isotopic analysis. Although not important for glass, under these experimental conditions, In, Ta and Re are co-eluted, partially eluted and not-eluted with the Cu fraction, respectively. Due to the close elution of Co and Cu, in practice 3 different fractions were collected for the actual glass samples: (i) 0–2.5 ml, (ii) 2.5–3 ml and (iii) 3–7 ml. Then, the Cu and Co contents were always measured in different fractions and the fractions were appropriately mixed whenever necessary to obtain quantitative Cu recovery prior to isotopic analysis. As for the in-house standard and the NIST SRM 610 glass reference material, quantitative recovery of Cu (100 ± 5%) was also obtained for the samples.
| Element | Recovery (%) | Element | Recovery (%) | Element | Recovery (%) |
|---|---|---|---|---|---|
| Li | 0 | Ge | 3.5 | Nd | 0 |
| Be | 0 | As | 0.1 | Sm | 0 |
| B | 2.1 | Rb | <0.1 | Gd | 0 |
| Na | 0 | Sr | 0 | Tb | 0 |
| Mg | 0.1 | Y | 0 | Dy | 0 |
| Al | 0.1 | Zr | <0.1 | Ho | 0 |
| P | 5.9 | Nb | 2.2 | Er | <0.1 |
| Ca | 0 | Mo | 0 | Tm | 0 |
| CK | 0 | Pd | 0 | Yb | 0 |
| Sc | <0.1 | Ag | 0 | Lu | 0 |
| Ti | <0.1 | Cd | <0.1 | Hf | <0.1 |
| V | 0.4 | In | 30.1 | Ta | 2.2 |
| Cr | 0.2 | Sn | 0.5 | W | 0 |
| Mn | <0.1 | Sb | <0.1 | Re | 18.9 |
| Co | 99.5 | Cs | 0.2 | Au | 0.4 |
| Ni | <0.1 | Ba | <0.1 | Tl | 0 |
| Cu | 100 | La | 0 | Bi | <0.1 |
| Zn | 0.1 | Ce | 0 | Th | <0.1 |
| Ga | 0 | Pr | 0 | U | 0 |
Cu isotopic analysis
Depending on the furnace atmosphere, Cu can impart different colours to the glass. This element was introduced in the glass melt either as metallic Cu (native or pure Cu or Cu alloy) or as a Cu-containing mineral.26Fig. 3 shows the δ65Cu values obtained for the glass samples after mass bias correction. Results obtained correspond to the mean value of two different measurements performed during the same session (measurement day). It can be seen that all glasses are depleted in δ65Cu with respect to NIST SRM 976. The samples from Sagalassos show a variation in δ65Cu ranging from −1.92 to −1.00‰, while the Mesopotamian samples range between δ65Cu from −1.12 to −0.35‰. The Pichvnari and Egyptian glasses show intermediate values (from −0.87 to −0.47‰). Since there is no evidence that smelting or refining alters the Cu isotopic composition of an ore,27 and hence, the Cu isotope ratio in a glass and in the corresponding Cu ore should be the same, Cu isotopic analysis shows promise as a tool for distinguishing provenances of Cu sources used in glass making, as was also concluded earlier for bronze samples.28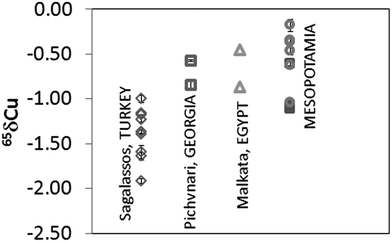 | ||
| Fig. 3 Cu isotope ratio results expressed in δ65Cu for glass samples. Measurements correspond to two measurements performed on the same day. (○: Nippur and □: Nuzzi, both in Mesopotamia). | ||
Unfortunately, natural fractionation of Cu is complicated. The redox reactions occurring during weathering of Cu minerals lead to different patterns in the isotopic signatures of ores.29 Primary sulfides are formed at high temperatures, whereas secondary carbonate/oxide phases are formed during low temperature supergene processes.30 During these processes, Cu fractionation occurs and it is assumed that Cu minerals precipitated from high temperature fluids will show much lower fractionation compared to minerals from low temperature precipitation (typically <30 °C).31 It has indeed been reported that Cu sulfides (primary ores) show lower δ65Cu values than Cu oxides.32–34 Also, some cases wherein higher δ65Cu values are found for sulfides (primary sources) than for oxides (secondary ores) from the same deposit can be found in the literature.30 Variations of about 3‰ can be found within a single deposit when comparing carbonates (secondary) and primary sulfides.34 Therefore, the link between a glass sample and the corresponding raw Cu-containg material source is complicated since sulfides, oxides or mixtures of both could have been used for glass production.
Sb isotopic analysis
Different to Cu, Sb is mostly present in nature as a sulfide, particularly as stibnite, Sb2S3. Sb oxides such as stibiconite (Sb3O6(OH)), valentinite (Sb2O3) or senarmontite (Sb2O3) are far less common. Therefore, sulfides are the most probable raw material source of Sb in ancient glass.Sb isotope ratio results were obtained for late Bronze Age Egyptian and Mesopotamian glasses. Not all samples were analysed for their Sb isotopic composition, as for some, the Sb content was too low (<0.05% Sb) and/or the small amount of sample available too low. Previously published ε123Sb-values for glass from Sagalassos and Georgia have been included here for comparison.16Fig. 4 shows all Sb isotope ratios obtained. Results correspond to the mean value and standard deviation of 2 measurements of the same sample performed during the same session (measurement day). The use of at least 2 different sources of Sb in Roman glasses has been suggested based on previous data. For the earlier (late Bronze Age to pre-Roman) glasses, such a hypothesis cannot be arrived at because the variation in Sb isotopic composition is considerably more narrow (∼1 ε123Sb units). Whereas Roman glasses have shown ε123Sb values between −1 and +3 ε123Sb for both coloured and colourless materials, Mesopotamian glasses show clearly negative values, ranging from −2.5 to −1.3 ε123Sb. The Egyptian and Pichvnari glasses exhibit similar variations in ε123Sb (from −1.5 to −0.44 ε) although their production took place in very different periods (14th century BC and 3rd–4th century BC). These results are situated in-between those for the Mesopotamian and Roman samples.
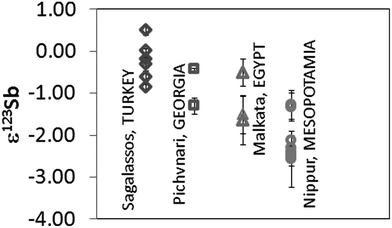 | ||
| Fig. 4 Sb isotope ratio results expressed in ε123Sb for glass samples. Measurements correspond to two measurements performed on the same day. | ||
It is not possible to assess whether one or more ore sources for Sb and Cu were used for the individual glass origins or periods. It can be observed however, that the range of values obtained for late Bronze Age Mesopotamian plant ash glasses are different from the Roman natron glasses from Sagalassos for both Sb and Cu. It can be inferred that the source(s) used in the manufacture of these glass types in different periods and using other technologies were different. While the range of Sb and Cu isotopic composition of the late Bronze Age Egyptian glasses is similar to that for the Mesopotamian material, the Pichvnari natron glass seems to occupy an intermediate position. This is an interesting observation, as this core-formed glass is in form and outlook very similar to the opaque late Bronze Age glass vessels, but the technology used relies on natron and not plant ash as a flux.
Conclusions
Based on the separation procedure proposed by Van Heghe et al.,18 isolation of Cu has been successfully applied to glass materials. In order to separate Cu and Co, 3.5 M HCl was used as a mobile phase, achieving quantitative recovery for both an in-house multi-elemental standard and the NIST SRM 610 glass reference material. Results obtained for glasses from late Bronze Age Mesopotamia and Egypt to the Greco-Roman world have shown that Cu variations range from δ65Cu = −1.9 up to −0.2‰. The link between the Cu isotopic composition in glass with its corresponding ore raw material, however, might be complex to reconstruct due to natural fractionation processes giving rise to different δ65Cu values in sulfides and carbonates/oxides from within the same ore deposit.For Sb, a smaller range of variation in its isotopic composition has been obtained for late Bronze Age glasses compared to Greco-Roman glass. This more narrow range in Sb composition may be due to the use of a single ore source for Sb as large scale recycling of glass was not common practice until Greco-Roman times. Although it is not possible to deduce whether one or more Cu or Sb sources were used for glass production of the samples investigated (based on previous work we presume that at least two Sb sources were used for Roman glass), it is reasonable to state that the raw materials used in late Bronze Age Mesopotamia and Egypt were different from those used in the Roman world.
Acknowledgements
Lara Lobo gratefully acknowledges the financial support received through Marie Curie Actions (FP7-PEOPLE-2010-IEF, project no. 273438). Frank Vanhaecke and Patrick Degryse acknowledge the financial support from the Flemish Research Foundation FWO-Vlaanderen under the form of a research project G002111N. Patrick Degryse holds an ERC Starting Grant ARCHGLASS, Grant agreement no. 240750. Richard Zettler and the University of Pennsylvania Museum of Archaeology and Anthropology in Philadelphia, USA, are acknowledged for the permission to sample the Nippur material. Joseph Greene, Adam Aja and the Harvard Semitic Museum are acknowledged for the permission to sample the Nuzi material.References
- A. J. Shortland and K. Eremin, Archaeometry, 2006, 48, 581–603 CrossRef CAS.
- Ancient glass: an interdisciplinary exploration, ed. J. Henderson, Cambridge University Press, New York, 2013, ch. 7, pp. 209–211 Search PubMed.
- Geomaterials in Cultural Heritage, ed. I. C. Freestone, The Geological Society, Special publication, UK, 2006, vol. 257, pp. 201–216 Search PubMed.
- A. J. Shortland, M. S. Tite and I. Ewart, Archaeometry, 2006, 48, 153–168 CrossRef CAS.
- Ancient Egyptian Materials and Technology, ed P. L. Nicholson and I. Shaw, Press Syndicate of the Universtity of Cambridge, Cambridge, 2000, p. 198 Search PubMed.
- G. D. Hatton, A. J. Shortland and M. S. Tite, J. Archaeol. Sci., 2008, 35, 1591–1604 CrossRef PubMed.
- M. Tite, T. Pradell and A. J. Shortland, Archaeometry, 2008, 50, 67–84 CrossRef CAS.
- N. Schibille, P. Degryse, M. Corremans and C. Specht, J. Archaeol. Sci., 2012, 39, 1480–1492 CrossRef CAS PubMed.
- C. M. Jackson, Archaeometry, 2005, 47, 763–780 CrossRef CAS.
- Lapis Lazuli from the kiln: Glass and Glass Making in the Late Bronze Age, ed A. Shortland, Leuven University Press, Leuven, 2012 Search PubMed.
- P. Degryse and P. Schneider, J. Archaeol. Sci., 2008, 35, 1993–2000 CrossRef PubMed.
- A. J. Shortland and H. Schroeder, Archaeometry, 2009, 6, 947–965 CrossRef.
- M. Ganio, S. Boyen, T. Fenn, R. Scott, S. Vanhoutte, D. Gimeno and P. Degryse, J. Anal. At. Spectrom., 2012, 27, 743–753 RSC.
- Isotopes in vitreous materials, ed. P. Degyrse, J. Henderson and G. Hodgins, Leuven University Press, Leuven, 2009 Search PubMed.
- L. Lobo, V. Devulder, P. Degryse and F. Vanhaecke, J. Anal. At. Spectrom., 2012, 27, 1304–1310 RSC.
- L. Lobo, P. Degryse, A. Shortland and F. Vanhaecke, J. Anal. At. Spectrom., 2013, 28, 1213–1219 RSC.
- C. N. Maréchal, P. Telouk and F. Albarede, Chem. Geol., 1999, 156, 251–273 CrossRef.
- L. Van Heghe, E. Engström, I. Rodushkin, C. Cloquet and F. Vanhaecke, J. Anal. At. Spectrom., 2012, 27, 1327–1334 RSC.
- F. Larner, M. Rehkamper, B. J. Coles, K. Kreissig, D. J. Weiss, B. Sampson, C. Unsworth and S. Strekopytov, J. Anal. At. Spectrom., 2011, 26, 1627–1632 RSC.
- S. Asaoka, Y. Takahashi, Y. Araki and M. Tanimizu, Anal. Sci., 2011, 27, 25–28 CrossRef CAS.
- D. C. Baxter, I. Rodushkin, E. Engstrom and D. Malinovsky, J. Anal. At. Spectrom., 2006, 21, 427–430 RSC.
- K. A. Kraus and G. E. Moore, J. Am. Chem. Soc., 1953, 75, 1460–1462 CrossRef CAS.
- C. N. Maréchal and F. Albarede, Geochim. Cosmochim. Acta, 2002, 66, 1499–1509 CrossRef.
- L. L. Lewis and W. A. Straub, Anal. Chem., 1960, 32, 96–99 CrossRef CAS.
- A manual of chemical analysis of metals, ed T. R. Dulski, Library of Congress Cataloging-in-Publication data, Ann Arbor MI, 1996, ch. 9, p. 119 Search PubMed.
- P. Mirti, P. Davit, M. Gulmini and L. Saguì, Archaeometry, 2001, 43, 491–502 CrossRef CAS PubMed.
- N. H. Gale, A. P. Woodhead, Z. A. Stos-Gale, A. Walder and I. Bowed, Int. J. Mass Spectrom., 1999, 184, 1–9 CrossRef CAS.
- E. Balliana, M. Aramendia, M. Resano, C. Brabante and F. Vanhaecke, Anal. Bioanal. Chem., 2013, 405, 2973–2986 CrossRef CAS PubMed.
- R. Mathur, J. Ruiz, M. J. Casselman, P. Megaw and R. van Egmond, Miner. Deposita, 2012, 47, 755–762 CrossRef CAS.
- R. Mathur, S. Titley, F. Barra, S. Brantely, M. Wilson, A. Phillips, F. Muzinaga, V. Maksaev, J. Vervoort and G. Hart, J. Geochem. Explor., 2009, 102, 1–6 CrossRef CAS PubMed.
- P. B. Larson, K. Maher, F. C. Ramos, Z. Chamg, M. Gaspar and L. D. Meinert, Chem. Geol., 2003, 201, 337–350 CrossRef CAS PubMed.
- S. Klein, C. Domergue, Y. Lahaye, G. P. Brey and H. M. von Kaenel, Journal of Iberian Geology, 2009, 35, 59–68 Search PubMed.
- S. Durali-Müller, Ph.D. thesis, University of Frankfurt, 2005.
- M. Haest, P. Muchez, J. C. J. Petit and F. Vanhaecke, Econ. Geol., 2009, 104, 1055–1064 CAS.
- P. Degryse, A. Boyce, N. Erb-Satullo, K. Eremin, S. Kirk, R. Scott, A. J. Shortland, J. Schneider and M. Walton, Archaeometry, 2010, 52, 380–388 CrossRef CAS.
- M. Walton, K. Eremin, A. Shortland, P. Degryse and S. Kirk, Archaeometry, 2012, 54, 835–852 CrossRef CAS.
- A. Shortland, N. Rogers and K. Eremin, J. Archaeol. Sci., 2007, 34, 781–789 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ja50303h |
| This journal is © The Royal Society of Chemistry 2014 |