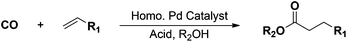Pd-catalyzed ethylene methoxycarbonylation with Brønsted acid ionic liquids as promoter and phase-separable reaction media†
Eduardo J.
García-Suárez
,
Santosh G.
Khokarale
,
Olivier N.
van Buu
,
Rasmus
Fehrmann
and
Anders
Riisager
*
Centre for Catalysis and Sustainable Chemistry, Department of Chemistry, Technical University of Denmark, DK-2800 Kgs. Lyngby, Denmark. E-mail: ar@kemi.dtu.dk; Fax: (+45) 45883136
First published on 12th September 2013
Abstract
Brønsted acid ionic liquids (BAILs) were prepared and applied as combined acid promoters and reaction media in Pd–phosphine catalyzed methoxycarbonylation of ethylene to produce methyl propionate. The BAILs served as alternatives to common mineral acids required for the reaction, e.g. methanesulfonic acid or sulfuric acid, resulting in high catalytic activity and selectivity towards methyl propionate. In addition, the BAILs yielded a biphasic system with the product and provided stability to palladium intermediates avoiding the undesirable formation of palladium black after reaction. These special features enabled facile methyl propionate separation and recovery of the ionic liquid catalyst system, thus allowing its re-use up to 15 times without apparent loss of catalytic activity or selectivity.
1. Introduction
Alkoxycarbonylation of olefins with carbon monoxide and alcohols is a versatile and atom-efficient C–C bond forming reaction (Scheme 1), which is applied industrially for the production of commodity alkyl esters and derivatives.1–3 The methoxycarbonylation of ethylene (Scheme 1, R1 = H and R2 = CH3) to obtain methyl propionate (MP) is of particular interest, due to the importance of MP as an intermediate in the production of methyl methacrylate (MMA) as a monomer applied to make poly-methylmethacrylate (p-MMA)4 – a transparent thermoplastic polymer in high demand with many useful applications such as alternative to glass, medical technologies, implants, etc.The methoxycarbonylation of ethylene is carried out efficiently under mild reaction conditions in the presence of Pd–phosphine complex catalysts, which afford high catalytic activity and product selectivity.5 Moreover, a rather strong Brønsted acid with pKa ≤ 4 (e.g. methanesulfonic acid (MSA), p-toluenesulfonic acid (TSA) or sulfuric acid (SA)) is needed to promote the reaction.6 The main roles of the acid are to preserve catalytic activity by facilitating protonation of catalytically inactive Pd(0) species into active [Pd(II)–H]+ species, and to stabilize intermediate cationic Pd(II) species formed during the catalytic cycle by weak coordination to the anions from the acid.6a,7 Major drawbacks of applying such acids are their manipulation, corrosion of reaction equipment and fast phosphine alkylation when using monodentate phosphines. Consequently, some attempts to avoid these drawbacks have been made by the employment of alternative acid promoters such as, e.g., polymeric sulfonic acids, borate esters, and aluminium triflate, instead of using common mineral acids.8
The use of ionic liquids (ILs) as reaction media in liquid–liquid biphasic reactions makes in many cases the processes greener than when using traditional organic solvents, due to the IL advantages such as low vapor pressure, good thermal stability, tunable solubility and acidity/coordination properties.9 Furthermore, ILs can also relatively easily be designed to accommodate functional groups which can provide the ILs with auxiliary reactivity like, e.g., Brønsted acidity.10 In line with this, Brønsted acid ionic liquids (BAILs) have been used successfully as alternative to mineral acids in many reactions.11
In this work, we introduce a versatile reaction concept for Pd–phosphine catalyzed methoxycarbonylation of ethylene to produce MP, where BAILs function as reaction media as well as alternative acid promoters to the commonly used strong acids (Fig. 1). The application of BAILs led to excellent results in terms of both catalytic activity and selectivity. Furthermore, the employed BAILs provide highly efficient immobilization of the palladium complex catalyst as well as a good stability of the catalytic Pd-intermediates. These features avoid the formation of palladium black and enable facile catalyst recovery and reutilization.
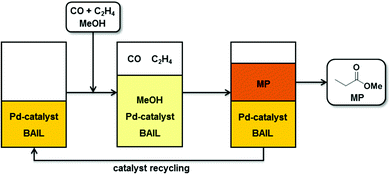 | ||
| Fig. 1 Schematic representation of ethylene methoxycarbonylation with BAILs as reaction media with MP product separation and Pd–catalyst recycling. | ||
2. Experimental
2.1 Materials
Triethylamine (≥99%), pyridine (≥99.8%), 1-methylimidazole (≥99%), methanesulfonic acid (MSA, ≥98%), p-toluenesulfonic acid (TSA, ≥98.5%), sulfuric acid (SA, 95–97%), triphenylphosphine (≥95%), 1,4-butanesultone (99.97%), methanol (≥99.8%), palladium acetate (99.98%), 1,2-bis(di-tert-butylphosphinomethane)benzene (DTBPMB, ≥98%) and 1-butyl-3-methylimidazolium methanesulfonate ([BMIm][MeSO3]) (≥95%) were purchased from Sigma-Aldrich and used without further purification. A gas mixture with a molar composition of CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C2H4
C2H4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ar = 2
Ar = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was purchased from AGA and used as received for the methoxycarbonylation reactions.
1 was purchased from AGA and used as received for the methoxycarbonylation reactions.
2.2 Synthesis and characterization of Brønsted acid ionic liquids (BAILs)
The employed BAILs were synthesized in two reaction steps following a slightly different procedure to that previously reported in the literature.12 The first reaction step involved the synthesis of zwitterions13 which were subsequently converted to BAILs by reaction with an equimolar amount of the corresponding acid (Scheme 2, see ESI† for details).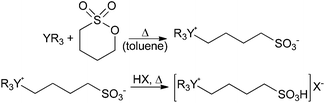 | ||
| Scheme 2 Synthesis route of the BAILs.12,13 YR3 = 1-methylimidazole, pyridine, triethylamine or triphenylphosphine; HX = methanesulfonic acid (MSA), p-toluenesulfonic acid (TSA) or sulfuric acid (SA). | ||
1H and 31P{1H} NMR spectra of the synthesized BAILs were recorded using either a Varian Mercury 300 MHz or a Varian Unity Inova 500 MHz spectrometer (ESI†).
The thermal stability of the BAILs was evaluated by thermal gravimetric analysis (TGA) using a Mettler Toledo (TGA/DSC1 STARe System) instrument under nitrogen flow (50 mL min−1) (ESI†). In a typical experiment the BAIL was heated from room temperature to 120 °C with a heating rate of 10 °C min−1. The sample was dried at this temperature for 2 h in order to eliminate moisture. Then, the sample was heated from 120 °C to 600 °C with a ramp rate of 10 °C min−1.
The relative acidity of the BAILs (and other applied Brønsted acids) was evaluated using a Cary 5000 UV-Vis spectrophotometer with 4-nitroaniline as the indicator according to reported procedures.14 In a typical experiment an ethanolic solution of 4-nitroaniline (0.1 mM) was added to a solution of the corresponding acid (10 mM) and the mixture was stirred overnight. Thereafter, the absorbance was measured and compared with the absorbance of a reference 4-nitroaniline solution. The absorbance difference was correlated to the Brønsted acidity through the Hammett acidity function H0 = pK(I) + log[IH+]/[I], where pK(I) is the pKa value of the indicator referred to an aqueous solution, and [I] and [IH+] are the molar concentrations of the un-protonated and protonated forms of the indicator, respectively.
2.3 Methoxycarbonylation of ethylene with BAILs
Catalytic experiments were performed in a 50 mL stainless steel Parr reactor equipped with a pressure transducer (Parr 4843). In a typical experiment palladium(II) acetate (11.2 mg, 0.05 mmol, 0.3 mol% Pd), 1,2-bis(di-tert-butylphosphinomethyl)benzene, DTBPMB (98.7 mg, 0.25 mmol, ligand/Pd molar ratio of 5) and 6 mL of a solution of the corresponding BAIL in MeOH (32 wt%) were introduced into the reactor directly or after stirring for 2 h under Ar at 80 °C in order to pre-activate the catalytic system. Afterwards, the reactor was flushed three times with the gas mixture of CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C2H4
C2H4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ar, pressurized to 20 bars and heated to 80 °C where the reaction was carried out. The conversion of the reactants was followed and correlated to the pressure drop of the reaction mixture after pre-calibration of the pressure transducer. After the reaction, the reactor was cooled down, depressurized and the product was analyzed by GC-FID (Agilent, 6890N, DB-1 capillary column, 50 m × 0.320 mm) to confirm the high purity of the formed MP.
Ar, pressurized to 20 bars and heated to 80 °C where the reaction was carried out. The conversion of the reactants was followed and correlated to the pressure drop of the reaction mixture after pre-calibration of the pressure transducer. After the reaction, the reactor was cooled down, depressurized and the product was analyzed by GC-FID (Agilent, 6890N, DB-1 capillary column, 50 m × 0.320 mm) to confirm the high purity of the formed MP.
For the recycling experiments the reactor was re-pressurized with the gas mixture up to 20 bars after cooling and depressurizing, as described above. After every fifth reaction run the MP phase was removed and fresh 5 mL MeOH was added.
3. Results and discussion
3.1 Synthesis and characterization of BAILs
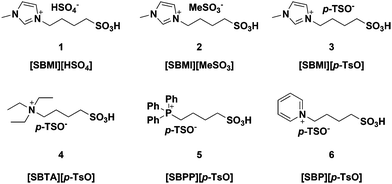
Six different BAILs consisting of different cations bearing an alkylsulfonated moiety and anions were prepared following a slightly modified reported procedure (Fig. 2).12 The purity of the prepared BAILs was confirmed by NMR spectroscopy and the thermal stability was measured by TGA (see ESI†). In addition, calculation of the Hammett acidity function of the BAILs was carried out in order to validate their acidic properties.The TGA profiles confirmed that the synthesized BAILs were thermally stable up to their decomposition temperature of 280–310 °C. No direct relationship was found between the decomposition temperature and the cation and/or anion composition. However, the phosphonium based BAIL [SBPP][p-TsO] (5) proved to be more thermally stable (Td = 310 °C) than the nitrogen based analogues, as also reported in the literature.15
The Hammett method consists of the determination of acidity functions using UV-Vis spectroscopy, where a basic indicator is used to trap the dissociative proton.14 In this work, 4-nitroaniline was selected as an indicator and ethanol was used as a solvent since most of the prepared BAILs were soluble herein. A maximum absorbance (Amax) of 1.65 was observed at λmax = 370 nm in ethanol for the un-protonated form of 4-nitroaniline. This absorbance decreased gradually when the concentration of the BAILs was increased, thus allowing the Hammett acidity function (H0) to be calculated from the ratio of the measured absorbances of the unprotonated ([I]) and protonated forms ([IH+]) of 4-nitroaniline (Table 1).
| Entry | Material |
A
max![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b |
[I] (%) | [IH+] (%) | H 0 |
|---|---|---|---|---|---|
| a H 0 = pK[I]aq + log([I]s/[IH+]s). 4-Nitroaniline and the BAILs were dissolved in ethanol with 0.1 mM and 10 mM, respectively. b Average absorbance at λ = 370 nm of three measurements. | |||||
| 1 | — | 1.65 | 100.0 | 0.0 | — |
| 2 | MeSO3H | 1.52 | 92.2 | 7.8 | 2.06 |
| 3 | H2SO4 | 1.51 | 91.5 | 8.5 | 2.02 |
| 4 | p-TsOH | 1.49 | 90.9 | 9.1 | 1.99 |
| 5 | [SBMI][HSO4] (1) | 1.54 | 93.2 | 6.8 | 2.12 |
| 6 | [SBMI][p-TsO] (3) | 1.53 | 93.0 | 7.0 | 2.11 |
| 7 | [SBTA][p-TsO] (4) | 1.52 | 92.1 | 7.9 | 2.06 |
| 8 | [SBPP][p-TsO] (5) | 1.53 | 93.0 | 7.0 | 2.11 |
| 9 | [BMIm][MeSO3] | 1.64 | 99.4 | 0.6 | 3.22 |
The Hammett acidity functions (H0) of the examined BAILs 1 and 3–5 (2 and 6 were not soluble enough in ethanol to allow the determination) were found to be quite similar, in the range of 2.06–2.12, thus suggesting only a minor influence of the anion and/or cation backbone structure on acidity. In contrast, the non-functionalized IL 1-butyl-3-methylimidazolium methanesulfonate ([BMIm][MeSO3]) revealed an H0 of 3.22 with an Amax value of 1.64, which was very close to the absorbance measured for the indicator 4-nitroaniline alone (1.65). This showed that the non-functionalized IL possessed very poor acidity, thus confirming the acidity of the BAILs to be correlated to the –SO3H group functionalization, as also expected.
3.2 Methoxycarbonylation of ethylene with BAILs
The prepared BAILs 1–6 were tested as combined acid promoters and reaction media in the methoxycarbonylation of ethylene for producing MP (32 wt% in methanol). For comparison the non-functionalized IL [BMIm][MeSO3] was also tested as reaction medium to demonstrate the decisive role of acid functionalization of the ILs (i.e. –SO3H group) in their successful application in the reaction. Similarly, the mineral acid MeSO3H was used as an acid promoter in catalytic amounts instead of the BAILs to benchmark the performance of the BAILs. In all reactions Pd(OAc)2 was selected as the catalyst precursor in combination with the diphosphine ligand DTBPMB, which has been reported to result in highly selective and active methoxycarbonylation systems for MP production.5a The obtained results are compiled in Table 2.| Entry | Acid promoter | H 0 | Conversion (%) | MP selectivity (%) |
|---|---|---|---|---|
a Reaction conditions: 0.05 mmol Pd(OAc)2 (0.3 mol% Pd), DTBPMB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd mol ratio = 5 Pd mol ratio = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6 mL of a 32 wt% solution of BAIL or IL in methanol, P(CO 1, 6 mL of a 32 wt% solution of BAIL or IL in methanol, P(CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C2H4 C2H4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ar = 2 Ar = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) = 22 bars, T = 80 °C, t = 20 min.
b With 5 equivalents of MeSO3H (acid 1) = 22 bars, T = 80 °C, t = 20 min.
b With 5 equivalents of MeSO3H (acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd mol ratio = 5 Pd mol ratio = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) instead of the BAIL, t = 10 min.
c With 1.2 equivalents of DTBPMB.
d
t = 120 min. 1) instead of the BAIL, t = 10 min.
c With 1.2 equivalents of DTBPMB.
d
t = 120 min.
|
||||
| 1b,c | MeSO3H | 2.06 | 99.2 | >99 |
| 2b | MeSO3H | 2.06 | 98.7 | >99 |
| 3 | [SBMI][HSO4] (1) | 2.12 | 95.3 | >99 |
| 4 | [SBMI][MeSO3] (2) | — | 99.2 | >99 |
| 5 | [SBMI][p-TsO] (3) | 2.11 | 99.1 | >99 |
| 6 | [SBTA][p-TsO] (4) | 2.06 | 99.2 | >99 |
| 7 | [SBPP][p-TsO] (5) | 2.11 | 98.3 | >99 |
| 8 | [SBP][p-TsO] (6) | — | 98.7 | >99 |
| 9d | [BMIm][MeSO3] | 3.22 | <1 | >99 |
Firstly, methoxycarbonylation of ethylene was performed using MeSO3H (5 equivalents) as an acid promoter in the presence of 1.2 or 5 equivalents of DTBPMB ligand (Table 2, entries 1 and 2). In both cases very high catalytic activity was obtained and a conversion of about 99% was achieved after 10 min of reaction with no apparent difference in reactivity pattern. However, a significant difference in the visual appearance of the post-reaction mixtures was indeed observed, as depicted in Fig. 3. When only 1.2 equivalents of the ligand were used a large amount of Pd-black was obviously formed, thus confirming the ligand amount to be insufficient to stabilize catalytically active palladium species. On the other hand, formation of Pd-black seemed to be avoided when 5 equivalents of the ligand were used and a clear yellow solution was obtained. Based on these findings, reaction conditions with 5 equivalents of the diphosphine ligand were selected for further experiments with the BAILs.
 | ||
Fig. 3 Reaction mixtures after Pd–DTBPMB catalyzed ethylene methoxycarbonylation with MeSO3H as an acid promoter and DTBPMB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd ratio = 1.2 Pd ratio = 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (left) and DTBPMB 1 (left) and DTBPMB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pd ratio = 5 Pd ratio = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (right). 1 (right). | ||
In the reactions where the BAILs were used as acid promoters in place of MeSO3H the reaction rates were somewhat lower, but excellent conversions of about 99% were still achieved in only 20 min (Table 2, entries 3–8) and – very importantly – the selectivity to MP remained higher than 99% (i.e. ≥98% MP yields). The observed activity difference cannot be correlated to the Brønsted acidity of the BAILs and MeSO3H, which were almost identical. Instead, the lower reaction rates were likely an effect of the lower solubility of the reactant gases in the BAIL–MeOH systems compared to pure MeOH, as normally observed in biphasic IL reaction systems,9 or the interference of the BAILs with the catalyst system. However, no influence on the catalytic activity of the cation structure and/or anion of the employed BAILs could directly be confirmed under the studied reaction conditions.
The non-functionalized IL [BMIm][MeSO3] was also tested as reaction medium (in the absence of acid promoter) under comparable reaction conditions. As expected, almost no conversion was achieved after 120 min of reaction (Table 2, entry 9). This demonstrates clearly that functionalization of the ILs with a strong acidic moiety, such as a sulfonic acid group, has a pivotal influence on the catalytic performance of the system under the selected reaction conditions.
One of the most important issues – and a common challenge in homogeneous catalysis – is the recovery and re-use of the catalytic system.16 In the reaction concept introduced in this study the role of the applied BAILs was not only to act as an acid promoter, but also to provide facile separation of the MP product by phase-separation and to preserve the catalyst solvation (see Fig. 1).
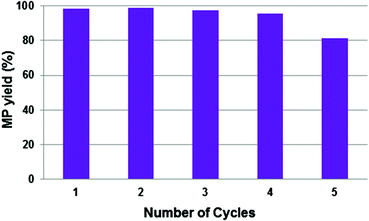
With this consideration in mind, the recyclability of the catalytic system with [SBMI][p-TSO] (3) was tested as a representative example of all the BAIL systems. The recycling experiments were carried out under the same reaction conditions used in the previous reactions, and the results are shown in Fig. 4.
As shown in Fig. 4, the catalytic system could be re-used four times with intermediate pressurizing of the reactor without any apparent loss of activity. However, after the fifth reaction run the activity was somewhat lowered due to Pd-black formation (Fig. 5a). We believe that the interaction of Pd(OAc)2 with the reactants – especially with CO which is a known reducing agent for homogeneous catalysts leading to metal precipitation17 – in the presence of the BAIL could enable Pd reduction or destabilization and further decomposition yielding Pd-black during the in situ complex formation. Notably, the reaction solution phase-separated after the fifth run (when a considerable amount of MP was formed) into an upper phase containing the MP and a lower phase containing the catalyst system dissolved in the BAIL, thus confirming the basis of the process concept to work.
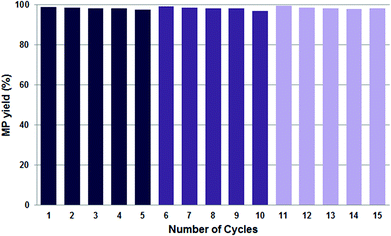
Instead of performing the complex formation in situ in the presence of the reactants, pre-formation of the catalytic system by stirring the BAIL, Pd(OAc)2 and DTBPMB ligand in MeOH under Ar for 2 h at 80 °C proved highly useful to avoid the formation of Pd-black and thus improve the reusability of the catalyst system. Hence, when the catalytic system was pre-formed it maintained its excellent performance of >97% MP yield during fifteen recycle experiments (Fig. 6), and after every fifth reaction the BAIL-catalyst system was recovered without the observation of any appreciable Pd-black (Fig. 5b–f) – or at least significantly less compared to the analogous reaction with the in situ formed catalyst system (Fig. 5a). This confirms that pre-formation of the catalytic system before mixing with the substrates (CO and ethylene) is essential to confer stability under the examined reaction conditions.
4. Conclusions
Efficient and durable Pd–diphosphine catalyst systems were prepared with BAILs and successfully applied in selective methoxycarbonylation of ethylene to obtain MP. Excellent results in terms of conversion and selectivity (>99% MP yield) were achieved. The application of BAILs allowed re-using the catalytic systems for fifteen times without any loss of performance, thus corroborating an efficient immobilization of the palladium complex catalyst. In addition, the use of BAILs provided a biphasic system with the MP product affording easy product separation and catalyst recovery – two features which are imperative for possible future industrial exploration.Acknowledgements
The Danish Council for Independent Research – Technology and Production Sciences (project no. 11-106979) has provided support for this work.References
- (a) B. Beller and A. M. Tafesh, in Applied Homogeneous Catalysis with Organometallic Compounds, ed. W. A. Hermann, Wiley-VCH, Weinheim, 1996 Search PubMed; (b) P. W. N. M. van Leeuwen, in Homogeneous Catalysis, Understanding the Art, ed. Kluwer Academic Publishers, Dordrecht, The Netherlands, 2004 Search PubMed; (c) Catalytic Carbonylation Reactions, Topics in Organometallic Chemistry, ed. M. Beller, Springer, Berlin, 2006 Search PubMed.
- (a) E. Drent and P. H. M. Budzelaar, Chem. Rev., 1996, 96, 663 CrossRef CAS PubMed; (b) R. A. M. Robertson and D. J. Cole-Hamilton, Coord. Chem. Rev., 2002, 225, 67 CrossRef CAS; (c) G. R. Eastham, R. P. Tooze, M. Kilner, D. F. Foster and D. J. Cole-Hamilton, J. Chem. Soc., Dalton Trans., 2002, 8, 1613 RSC; (d) A. Vavasori, G. Cavinato and L. Toniolo, J. Organomet. Chem., 2000, 601, 100 CrossRef; (e) C. Bianchini, A. Meli, W. Oberhauser, S. Parisel, O. V. Gusev, A. M. Kalsin, N. V. Vologdin and F. M. Dolgushin, J. Mol. Catal. A: Chem., 2004, 224, 35 CrossRef CAS PubMed; (f) C. Godard, B. K. Muñoz, A. Ruiz and C. Claver, Dalton Trans., 2008, 853 RSC.
- A. Brennführer, H. Neumann and M. Beller, ChemCatChem, 2009, 1, 28 CrossRef.
- (a) A. H. Tullo, Chem. Eng. News, 2009, 87(42), 22 CrossRef PubMed; (b) B. Harris, Ingenia, 2010, 45, 18 Search PubMed.
- (a) W. Clegg, G. R. Eastham, M. R. J. Elsegood, R. P. Tooze, X. L. Wang and K. Whiston, Chem. Commun., 1999, 1877 RSC; (b) J. M. D. Jeroen, D. Berth-Jan, J. E. Cornelis and K. V. Gerard, Adv. Synth. Catal., 2006, 348, 1447 CrossRef; (c) W. G. Reman, G. B. J. De Boer, S. A. J. Van Langen and A. Nahuijsen, Eur. Pat., EP 411 721 A3, 1989 Search PubMed (to Shell).
- (a) C. J. Rodriguez, D. F. Foster, G. R. Eastham and D. J. Cole-Hamilton, Chem. Commun., 2004, 1720 RSC; (b) G. P. C. M. Dekker, C. J. Elsevier, K. Vrieze, P. W. N. M. van Leeuwen and C. F. Roobeek, J. Organomet. Chem., 1992, 430, 357 CrossRef CAS; (c) B. A. Markies, D. Kruis, M. H. P. Rietveld, K. A. N. Verkerk, J. Boersma, H. Kooijman, M. Lakin, A. L. Spek and G. van Koten, J. Am. Chem. Soc., 1995, 117, 5263 CrossRef CAS.
- (a) C. Bianchini and A. Meli, Coord. Chem. Rev., 2002, 225, 35 CrossRef CAS; (b) M. A. Zuideveld, P. C. J. Kramer, P. W. N. M. van Leeuwen, P. A. A. Klusener, H. A. Stil and C. F. Roobek, J. Am. Chem. Soc., 1998, 120, 7977 CrossRef CAS; (c) E. Drent, P. Arnoldy and P. H. M. Budzelaar, J. Organomet. Chem., 1994, 475, 57 CrossRef CAS; (d) R. A. M. Robertson, A. D. Poole, M. J. Payne and D. J. Cole-Hamilton, Chem. Commun., 2001, 47 RSC.
- (a) H. Ooka, T. Inoue, S. Itsuno and M. Tanaka, Chem. Commun., 2005, 1173 RSC; (b) A. C. Ferreira, R. Crous, L. Bennie, A. M. M. Meij, K. Blann, B. C. B. Bezuidenhoudt, D. A. Young, M. J. Green and A. Roodt, Angew. Chem., Int. Ed., 2007, 46, 2273 CrossRef CAS PubMed; (c) T. O. Vieira, M. J. Green and H. Alper, Org. Lett., 2006, 8, 6143 CrossRef CAS PubMed; (d) D. Bradley, G. Williams, M. L. Shaw, M. J. Green and C. W. Holzapfel, Angew. Chem., Int. Ed., 2008, 120, 570 CrossRef.
- M. Haumann and A. Riisager, Chem. Rev., 2008, 108, 1474 CrossRef CAS PubMed.
- Ionic Liquids in Synthesis, ed. P. Wasserscheid and T. Welton, Wiley, 2007 Search PubMed.
- (a) F. Dong, C. Jian, F. Zhenghao, G. Kai and L. Zuliang, Catal. Commun., 2008, 9, 1924 CrossRef PubMed; (b) X. Lia, W. Eli and G. Lia, Catal. Commun., 2008, 9, 2264 CrossRef PubMed; (c) P. Wasserscheid, M. Sesing and W. Korth, Green Chem., 2002, 4, 134 RSC; (d) D. Li, F. Shi, S. Guo and Y. Deng, Tetrahedron Lett., 2004, 45, 265 CrossRef CAS PubMed; (e) Y. L. Gu, J. Mol. Catal. A: Chem., 2004, 71, 212 Search PubMed; (f) S. Saravanamurugan and A. Riisager, Catal. Today, 2013, 200, 94 CrossRef CAS PubMed; (g) J. C. Serrrano-Ruiz, J. M. Campelo, M. Francavilla, A. A. Romero, R. Luque, C. Menendez-Vazquez, A. B. Garcia and E. J. Garcia-Suarez, Catal. Sci. Tech., 2012, 9, 1828 RSC; (h) H. Zhang, F. Xu, G. Zhang and C. Wang, Green Chem., 2007, 9, 1208 RSC.
- A. C. Cole, J. L. Jensen, I. Ntai, K. L. T. Tran, K. J. Weaver, D. C. Forbes and J. H. Davis, J. Am. Chem. Soc., 2002, 124, 5962 CrossRef CAS PubMed.
- S. G. Khokarade, E. J. García-Suárez, J. Xiong, U. V. Mentzel, R. Fehrmann and A. Riisager, Catal. Commun., 2013 DOI:10.1016/j.catcom.2013.09.005.
- (a) C. Thomazeau, H. Olivier-Bourbigou, L. Magna, S. Luts and B. Gilbert, J. Am. Chem. Soc., 2003, 125, 5264 CrossRef CAS PubMed; (b) Z. Y. Du, Z. P. Li, S. Guo, J. Zhang and Y. Q. Deng, J. Phys. Chem. B, 2005, 109, 19542 CrossRef CAS PubMed.
- J. Luo, O. Conrad and I. F. J. Vankelecom, J. Mater. Chem., 2012, 22, 20574 RSC.
- D. J. Cole-Hamilton and R. P. Tooze, Catalyst Separation, Recovery and Recycling, Springer, Netherlands, 2006 Search PubMed.
- (a) G. R. Eastham, Studies on the palladium catalysed methoxycarbonylation of ethane, Durham theses, Durham University, 1998 Search PubMed; (b) C. Bianchini and A. Meli, Coord. Chem. Rev., 2002, 225, 35 CrossRef CAS; (c) P. W. N. M. van Leeuwen, Appl. Catal., A, 2001, 212, 61 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3gc41380b |
| This journal is © The Royal Society of Chemistry 2014 |