Quantitative assessment of inhalation exposure and deposited dose of aerosol from nanotechnology-based consumer sprays†
Yevgen
Nazarenko‡
a,
Paul J.
Lioy
bc and
Gediminas
Mainelis
*ac
aDepartment of Environmental Sciences, Rutgers University, 14 College Farm Rd., New Brunswick, NJ 08901, USA
bRobert Wood Johnson Medical School, Rutgers University, 170 Frelinghuysen Road, Piscataway, NJ 08854, USA
cRutgers Environmental and Occupational Health Sciences Institute, 170 Frelinghuysen Road, Piscataway, NJ 08854, USA. E-mail: mainelis@envsci.rutgers.edu; Fax: +1 732 932 8644; Tel: +1 848 932 5712
First published on 28th January 2014
Abstract
This study provides a quantitative assessment of inhalation exposure and deposited aerosol dose in the 14 nm to 20 μm particle size range based on the aerosol measurements conducted during realistic usage simulation of five nanotechnology-based and five regular spray products matching the nano-products by purpose of application. The products were also examined using transmission electron microscopy. In seven out of ten sprays, the highest inhalation exposure was observed for the coarse (2.5–10 μm) particles while being minimal or below the detection limit for the remaining three sprays. Nanosized aerosol particles (14–100 nm) were released, which resulted in low but measurable inhalation exposures from all of the investigated consumer sprays. Eight out of ten products produced high total deposited aerosol doses on the order of 101–103 ng kg−1 bw per application, ~85–88% of which were in the head airways, only <10% in the alveolar region and <8% in the tracheobronchial region. One nano and one regular spray produced substantially lower total deposited doses (by 2–4 orders of magnitude less), only ~52–64% of which were in the head while ~29–40% in the alveolar region. The electron microscopy data showed nanosized objects in some products not labeled as nanotechnology-based and conversely did not find nano-objects in some nano-sprays. We found no correlation between nano-object presence and abundance as per the electron microscopy data and the determined inhalation exposures and deposited doses. The findings of this study and the reported quantitative exposure data will be valuable for the manufacturers of nanotechnology-based consumer sprays to minimize inhalation exposure from their products, as well as for the regulators focusing on protecting the public health.
Nano impactThis study provides a quantitative assessment of human inhalation exposure to nanomaterials due to the use of nanotechnology-based consumer sprays. To the best of our knowledge, it is the first quantitative exposure assessment of a wide selection of consumer spray products in a realistic exposure scenario. We expect this study to generate a substantial impact due to high public interest and attention of the governmental and non-governmental agencies to the safety of nanotechnology-based consumer products. This study is published just as the Regulation of the European Parliament and of the Council on Cosmetic Products, PE-CONS 3623/09 (2009) came into legal effect in 2013 mandating reporting of “the reasonably foreseeable exposure conditions” for all of the nanomaterial-containing cosmetic products on the EU market. |
Introduction
The use of engineered nanomaterials in consumer products is gradually becoming pervasive. Depending on the geopolitical area, nanomaterials that are engineered but do not have a novel molecular identity and/or those manufactured in small to moderate quantities are not regulated by government agencies.1–5 This provides for an increasing share of common consumer products becoming nanotechnology-based, due to introduction of engineered nanomaterials into such products. The types of products where engineered nanomaterials may be or are used include cosmetics and personal care products, nutritional supplements and drugs, household and industrial use chemicals and antiseptics, all of which may be in the form of liquids or powders and can be easily dispersed into the air.6–12 Another category of nanotechnology-based products is solid products which cannot be dispersed, for example, electronics and equipment, structural materials, apparel, etc.When nanotechnology-based consumer products are manufactured, used and disposed, engineered nanomaterials may be released, which can lead to human and environmental exposure.13–18 Human exposure can occur through ingestion, inhalation or the cutaneous route.19 The inhalation exposure route has been identified as potentially the likeliest one for those consumer products which are or can easily be dispersed as aerosol during their normal use, such as sprays or powders.13,16,17,20–22
In our earlier study of quantitative exposures from consumer products, we investigated inhalation exposure from several cosmetic powders.23 Here, we report the results of a quantitative exposure assessment for selected consumer sprays, which are supposed to be used as antiseptics, cosmetics and personal care products. Ten spray products initially identified by Nazarenko et al.16 were investigated. The selection comprised five pairs of nanotechnology-based and non-nanotechnology-based (regular) sprays with each pair consisting of two products with the same purpose of application. Categorization was based on product labeling and/or marketing as nanotechnology-based or non-nanotechnology-based (regular). When the liquid products are sprayed (used) close to the personal breathing zone, they will generate aerosol particles containing engineered nanomaterials if they are present in the original products. Engineered nanomaterials may be distributed in the generated aerosol in complex ways because of agglomeration.24–28 At the same time, we found that wide aerosol size distributions were formed due to spraying of both the nanotechnology-based and regular consumer spray products, including production of nanosized particles as well as super-micron agglomerates by all products.16 These findings confirmed the potential for nanomaterial exposure from particles within a wide range of sizes. The engineered nanomaterials are likely distributed across both the nanosized aerosol fraction (<100 nm) and larger particles in an agglomerated form, sometimes as large as 20 μm, which was the upper limit of our measuring equipment. However, two things remain unknown: (1) the exact quantities of aerosol particles of different sizes that are inhaled and (2) how much aerosol is deposited in various regions of the human respiratory system, thereby potentially delivering engineered nanomaterials into the body via the inhalation route. Such quantitative exposure information is crucial for risk assessment and studies investigating health effects of nanomaterial exposure.
The quantitative investigation of inhalation exposure presented here will be valuable for the manufacturers of nanotechnology-based sprays because it provides insights into how such an exposure occurs and what factors influence its magnitude, which may help formulate the products and design sprayers in a way that will minimize generation of aerosol particles of unwanted sizes. Lastly, this paper provides quantitative exposure data for real consumer spray products acquired from the market which we hope will help in risk assessment and development of any consumer-oriented regulations and/or guidelines.
Materials and methods
Summary
All nanotechnology-based and regular consumer spray products were investigated using transmission electron microscopy (TEM) in order to determine the size, shape, state of agglomeration, and electron beam sensitivity of the particles in them. Where particulate matter was observed, we took micrographs at various magnifications. We then reprocessed the original aerosol size distribution measurement data collected in an earlier study16 for use in the exposure calculations presented here. In that 2011 study, aerosol size distributions were measured when the sprayers containing the original product (when available) were activated manually in the immediate vicinity of a human mannequin head to simulate use by a consumer. The released particles were sampled through stainless steel tubes inserted in the nostrils of the mannequin head to simulate potential inhalation exposure. For this study, the measurement data from Nazarenko et al.16 were exported from the aerosol instrument manager software (AIM Manager, TSI, Inc., Shoreview, MN, USA) as aerosol particle mass distributions, which were then used in a mathematical model to calculate the masses of “inhaled” and “deposited” particulate matter.Investigated products
The consumer spray products, for which exposure was assessed quantitatively and reported herein, had been described in an earlier study.16 The selected sprays, for which aerosol size distributions were measured, included 5 products marketed as nanotechnology-based and 5 regular products. These products, along with their compositions reproduced verbatim from the product labels, are listed in Table 1. The brand names of the products were substituted with descriptive names according to the purpose of application. The five regular products matched the five nanoproducts by their purpose of application and included a pair of topical antimicrobial silver sprays, a pair of facial cosmetic sprays, a pair of hair sprays, a pair of surface disinfectant sprays, and a pair of skin hydrating sprays.| Product | Compositiona |
|---|---|
| a Reproduced from the product labels exactly. | |
| Silver nanospray | Silver nanoparticles, purified water |
| Regular silver spray | 99.99% Pure silver suspended in demineralized water |
| Facial nanospray | Distilled water, vitamin C, nanosize particles of copper, calcium, magnesium and zinc |
| Regular facial spray | Water, butylene glycol, glycerin, panthenol, tocopheryl acetate, phenoxyethanol, alcohol denat., methylparaben, lecithin, Rosa centifolia (rose) water, butylparaben, ethylparaben, isobutylparaben, propylparaben |
| Hair nanospray | Alcohol denat., aqua, PVP/VA co-polymer, isopropyl alcohol, myrtrimonium bromide, parfum |
| Regular hair spray | SDA alcohol 40-B, water, VA/crotonates/vinyl neodecanoate co-polymer, octylacrylamide/acrylates/butylaminoethyl methacrylate co-polymer, aminomethanol propanol, lauryl pyrrolidone, PEG-75 lanolin, cyclopentasiloxane, fragrance |
| Disinfectant nanospray | Parachlorometaxylenol – 0.20%, other ingredients – 99.80% |
| Regular disinfectant spray | o-Phenylphenol – 0.22%, diisobutylphenoxyethoxy ethyl dimethyl benzyl ammonium chloride monohydrate – 0.70%, inert ingredients – 99.08% |
| Skin hydrating nanomist | Purified water, dimethicone, copolyol, algae extract, mugwort (Artemisia vulgaris) extract, Aloe barbadensis gel, fucogel, plankton extract, lavender (Lavendula angustifolia) oil, calcium PCA, zinc PCA, phenoxyethanol, methylparaben, propylparaben |
| Regular skin hydrating mist | Water, glycerin, hyaluronic acid, diazolidinyl urea, polysorbate 80, ergothioneine, Aloe barbadensis leaf juice, sodium carboxymethyl b-glucan, Camellia sinensis leaf extract, tetrasodium EDTA, allantoin, citrus Aurantium bergamia (bergamot) fruit oil, citric acid, kinetin, iodopropynyl butylcarbamate |
TEM characterization of consumer sprays
A small quantity of each liquid consumer spray was spread on an HC300-Cu TEM grid (Electron Microscopy Sciences, Hatfield, PA, USA) using a glass stick and allowed to dry at room temperature (22–23 °C) and humidity (15–35% RH) for at least 24 hours. A transmission electron microscope (2010F, JEOL Ltd, Tokyo, Japan) in the TEM mode was used to examine these specimens at different magnifications. Digital micrographs with automatically inserted scale bars were taken. For the silver particles where the atomic grid could be observed, the corresponding micrographs were amended with small image insets showing it.Simulated use of consumer sprays
The methodology for simulated use of the consumer sprays and aerosol sampling is described in detail elsewhere.16 Briefly, we placed a human mannequin head inside a level II biosafety cabinet (NUAIRE, Inc., Plymouth, MN, USA), which is equipped with a high-efficiency particulate air (HEPA) filtration system. The biosafety cabinet was furnished with a polyethylene curtain covering the front opening of the cabinet, in which gloves for manual activation of the sprays were fitted. Products were sprayed in close proximity of the mannequin head, but the spray cone was directed towards the back wall of the cabinet, in the same direction where the mannequin head was facing. Thus, the products were not sprayed directly into the face of the mannequin but in close proximity. The mannequin head had two stainless steel tubes inserted into its nostrils. The two stainless steel tubes exited the head at the nape where they were joined by a stainless steel Y-connector, and the combined aerosol stream passed through conductive tubing into a stainless steel flow splitter and then into the aerosol measurement instruments: a Scanning Mobility Particle Sizer (SMPS) (module combination 3080/3786, TSI, Inc.) and an Aerodynamic Particle Sizer (APS) (model 3321, TSI, Inc.). These instruments measured aerosol size distributions in the size range ~14 nm to 20 μm. Three replicates were carried out for each consumer spray. We exported the measurement data using the AIM software (TSI, Inc.) as aerosol particle mass concentrations assuming a spherical shape of particles and a particle density of 1 g cm−3. The justification for using this particle density has been provided previously.16 The results of the dose assessment reported herein can be adjusted for a different particle density, if such data become available.This configuration of the experimental setup allowed measurement of released aerosol particles from the simulated personal breathing cloud. Therefore, we assumed that the concentrations and size distributions of the measured particles are representative of those inhaled during the actual spray application by consumers.
Quantitative exposure assessment
The mass-based aerosol concentrations across the measurement size range ~14 nm to 20 μm were used as input in the mathematical model that had been used earlier for the quantitative exposure assessment of cosmetic powders.23 Similar to that earlier study, the “inhalation exposure” and “deposited dose” were calculated. “Inhalation exposure” is the aerosol mass entering the human respiratory system during an exposure event. It was calculated separately for several aerosol particle size ranges indicated as subscripts in μm: PM0.1–0.014 (ultrafine aerosol fraction), PM1–0.1 (submicron fraction of fine particles), PM2.5–1 (micron fraction of fine particles), PM10–2.5 (coarse particles), and PM20–10 (supercoarse particles29). “Deposited dose” is the aerosol mass of all measured particle sizes deposited in the human respiratory system during an exposure event. The deposited dose was calculated for the entire respiratory system and individually for each region of the respiratory system: the head airways (HA), the tracheobronchial region (TB) and the alveolar region (AL).The detailed description of the employed mathematical model and its development was published earlier.23 We are also providing the equations and definitions of this model in the ESI (Supplementary Methods).† The same user profile reported by Nazarenko et al.23 was used for the consumer sprays: inhalation flow rate (Qinh) = 11.0 L min−1 and body weight (bw) = 60 kg, which correspond to the body weight and breathing rate suggested for assessing short-term exposures of 18–60 year old females performing light activities.30 The justification of this choice of user and activity profile was discussed earlier.23
The duration of each exposure event was assumed to be Tcontact = 1 min; however, a direct adjustment of this duration may be done to calculate the inhalation exposure of different durations as required by any other exposure scenario.
Similar to the case with cosmetic powders in our previous study,23 here we also could not obtain information about the amount or fraction of nanomaterials in the investigated consumer sprays in order to determine fnano. As in the cosmetic powder study, we assumed fnano = 1 indicating that aerosolized particles produced from the nanotechnology-based products consist completely (100%) of nanomaterials (the worst case scenario). However, the dose calculations can be easily adjusted should nanomaterial content become known. We assumed aerosol particle losses in the sampling lines as negligible.
The deposited dose was determined as aerosol mass deposited in each of the three regions of the respiratory system and in the entire respiratory system during a 1-minute exposure event per 1 kg of body weight in the same way as in the previous study for cosmetic powders.23
As in the previous study,23 we also determined the deposited dose for the HA, TB and AL regions of the respiratory system as percentage of the total deposited dose. This presentation allowed us to illustrate the respiratory system region with the highest deposited dose relative to the other regions.
Results
TEM characterization of consumer sprays
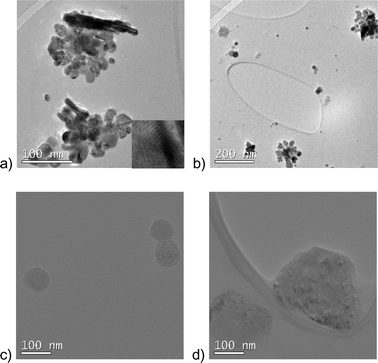
Fig. 1 and 2 demonstrate two selected TEM micrographs for each of the consumer sprays, for which the TEM investigation showed presence of particles or electron-contrast structures. These include two nanotechnology-based silver nanospray and disinfectant nanospray and five non-nanotechnology-based regular silver spray, regular disinfectant spray, regular hair spray, regular skin hydrating mist, and regular facial spray. In three nanotechnology-based products (facial nanospray, hair nanospray and skin hydrating nanomist), no such particles or structures were observed.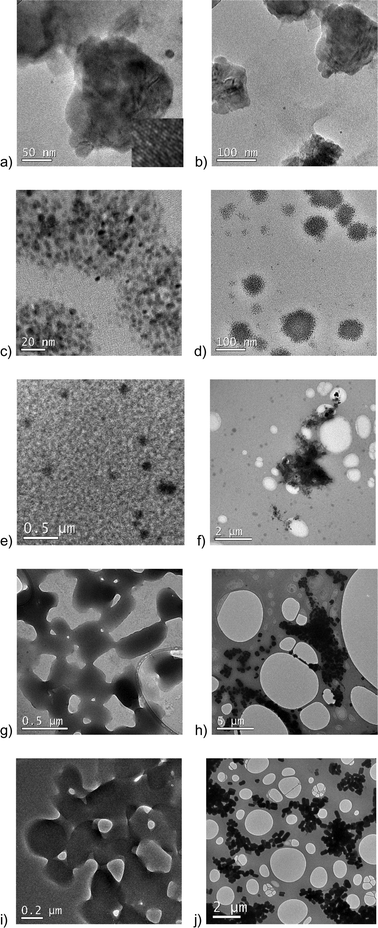 | ||
| Fig. 2 TEM micrographs of regular silver spray (a, b), regular disinfectant spray (c, d), regular hair spray (e, f), regular skin hydrating mist (g, h), and regular facial spray (i, j). | ||
The phenomenon of radiolysis (alteration of a material by the energy of the electron beam) above a certain magnification was observed for two regular products: regular skin hydrating mist and regular facial spray. This phenomenon has been described earlier.16,31 It can be seen that the particles with clear boundaries as seen in Fig. 2h and j at low magnifications, when the diameter of the electron beam is larger, “melted” when viewed at higher magnifications (Fig. 2g and i), when we focused the electron beam in a smaller area of the sample. This is likely an indication of the organic chemical nature of the particles in these two products.
In the silver nanospray (Fig. 1a and b) and the regular silver spray (Fig. 2a and b), we found silver nanoparticles with sizes from ~3 nm to several dozen nanometers and larger. Most particles were agglomerated, especially in the silver nanospray. The silver nanospray contained on average smaller particles than its regular counterpart. In the regular silver spray, we also observed particles and agglomerates larger than 100 nm (up to almost 0.5 μm).
The disinfectant nanospray (Fig. 1c and d) presented only a few low-contrast particles with a very low level of agglomeration. The smallest particles were in the nanosize range (about 70 nm), while the largest particles were slightly larger than 200 nm. The low electron contrast of these particles may be an indication of their organic nature or that they are composed of lighter chemical elements.
The regular disinfectant spray presented a very interesting particle structure as shown in Fig. 2c and d: spherical particles, most of which were below 100 nm in size and looked either nanostructured or as agglomerates of very small nanoparticles (1–2 nm). The electron contrast of these 1–2 nm nanoparticles or nanostructure elements varied from low to high. There is also an additional level of agglomeration of the larger nanostructured or agglomerated particles. They were observed attached to each other in pairs and up to several particles.
The regular hair spray (Fig. 2e and f) had two different kinds of particles, some of which were nanosized (as small as ~16 nm). The particles of the first kind were small spherical and of varying sizes (~16 to above 100 nm). The size of agglomerates reached almost 700 nm. There were also areas containing loosely associated agglomerates extending to 2–3 μm in size.
The TEM micrographs of the regular skin hydrating mist (Fig. 2g and h) and regular facial spray (Fig. 2i and j) looked very similar. However, the smallest visible particles in the regular facial spray were in the nanosize range (as small as ~80 nm), while the smallest particles in the regular skin hydrating mist were ~150 nm. The largest particles in these two sprays were very large compared with the other investigated sprays: >2.5 μm in the regular skin hydrating mist and >6 μm in the regular facial spray. As described above, the particles in these two products were altered due to radiolysis at higher magnifications of the TEM.
Quantitative exposure assessment
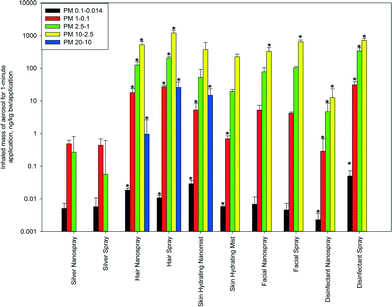 | ||
| Fig. 3 Inhalation exposure of PM from consumer sprays based on mass concentration of particulate matter in different aerosol particle size fractions sampled with the mannequin head sampler during simulated product application. The data represent averages of three repeats. Error bars represent one standard deviation. * denotes a significant difference (p < 0.05) for a given particle size fraction between a nano and a regular product, based on the two-tailed Student's t test. Numerical data used to produce the figure are provided in the ESI,† Table S1. | ||
It can be seen that the inhalation exposure of the ultrafine aerosol fraction (PM0.1–0.014) was in a wide range between ~0.002 (disinfectant nanospray) and ~0.05 (disinfectant spray) ng kg−1 bw per application. Disinfectant nanospray, facial spray, silver nanospray, skin hydrating mist, silver spray, and facial nanospray produced the lowest inhalation exposures (~0.002–0.007 ng kg−1 bw per application). Hair spray, hair nanospray, skin hydrating nanomist, and disinfectant spray produced inhalation exposures that were approximately an order of magnitude higher (~0.01–0.05 ng kg−1 bw per application).
In the submicron fraction of fine particles (PM1–0.1), a relatively wide range of inhalation exposure was observed for different sprays: from ~0.3 (disinfectant nanospray) to ~31 (disinfectant spray) ng kg−1 bw per application. The use of four products (disinfectant nanospray, silver spray, silver nanospray, and skin hydrating mist) resulted in a very low PM1–0.1 below 1 ng kg−1 bw per application. Three products (facial spray, facial nanospray and skin hydrating nanomist) produced inhalation doses of PM1–0.1 around ~4–5 ng kg−1 bw per application. Three products (hair nanospray, hair spray and disinfectant spray) produced relatively very high PM1–0.1 inhalation exposures of ~18–31 ng kg−1 bw per application.
The inhalation exposure of the micron fraction of fine particles (PM2.5–1) varied greatly as well: from ~0.06 (silver spray) to ~340 (disinfectant spray) ng kg−1 bw per application. Silver spray and silver nanospray produced PM2.5–1 inhalation exposure below 1 ng kg−1 bw per application: ~0.06 and ~0.3, respectively. Higher PM2.5–1 inhalation exposures ranging between 1 and 100 ng kg−1 bw per application were produced by disinfectant nanospray (~4), skin hydrating mist (~20), skin hydrating nanomist (∼53), and facial nanospray (~78). The highest PM2.5–1 inhalation exposures exceeding 100 ng kg−1 bw per application were produced by facial spray (~103), hair nanospray (~126), hair spray (~205), and disinfectant spray (~340).
No coarse particles (the PM10–2.5 fraction) and, hence, no corresponding inhalation exposures to coarse particles were determined in two out of ten products: silver nanospray and silver spray. The PM10–2.5 inhalation exposure was the lowest for disinfectant nanospray (~13 ng kg−1 bw per application) and highest for hair spray (1200 ng kg−1 bw per application). For the other consumer sprays, the PM10–2.5 inhalation exposure ranged between approximately 200 and 700 ng kg−1 bw per application.
Particles above 10 μm and, accordingly, inhalation exposure of PM20–10 (supercoarse particles)29 were detected in only three products: ~1 ng kg−1 bw per application for hair nanospray, ~15 ng kg−1 bw per application for skin hydrating mist and ~26 ng kg−1 bw per application for hair spray.
Based on the two-tailed Student's t test assuming equal variance, the inhalation exposures were statistically different: (1) in all aerosol particle size fractions for the nano and regular hair sprays and for the nano and regular disinfectant sprays, (2) in all but the PM10–2.5 size fraction for the nano and regular skin hydrating mists, and (3) in the PM10–2.5 fraction only for the nano and regular facial sprays.
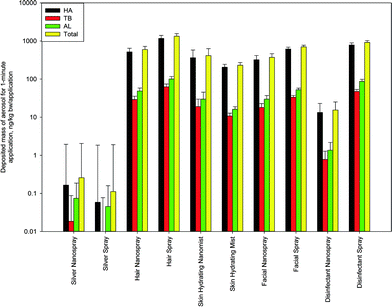 | ||
| Fig. 4 Deposited dose of PM from consumer sprays deposited in different regions of the respiratory system (the head airways (HA), the tracheobronchial (TB) and the alveolar (AL) regions). The data represent averages of three repeats. Error bars represent one standard deviation and illustrate uncertainty of model results propagating from known uncertainty of experimental data. Numerical data used to produce the figure are provided in the ESI,† Table S2. | ||
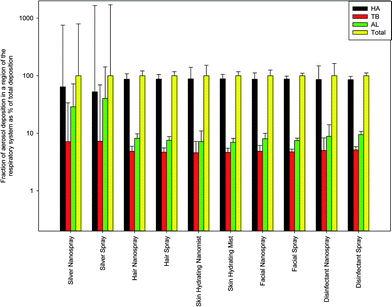 | ||
| Fig. 5 Percent distribution of PM from consumer sprays deposited in different regions of the respiratory system (the head airways (HA), the tracheobronchial (TB) and the alveolar (AL) regions). The data represent averages of three repeats. Error bars represent one standard deviation and illustrate uncertainty of model results propagating from known uncertainty of experimental data. Numerical data used to produce the figure are provided in the ESI,† Table S3. | ||
Discussion
This study found that the release of aerosol particles in various size fractions from different consumer spray products varied greatly from product to product. This is in contrast to the results of our previous study focusing on cosmetic powders, where relatively similar proportions of the concentrations in different particle size fractions were observed for various powders.23 For easier comparison, the ranges of inhalation exposure and deposited doses for the sprays and the powders are summarized in Table 2. The high variability of aerosol size distributions among consumer spray products suggests substantially different exposure levels to different particle size fractions depending on the product.| Property | Sprays (ng kg−1 bw per application) | Powdersa (ng kg−1 bw per application) |
|---|---|---|
| a Nazarenko et al.23 | ||
| Range of total inhalation exposure | 5.0 × 10−1–1452.6 | 38.7–33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 317.7 317.7 |
| Range of inhalation exposure to PM0.1–0.014 | 2.3 × 10−3–5.0 × 10−2 | 0–5.7 × 10−3 |
| Range of inhalation exposure to PM1–0.1 | 2.9 × 10−1–31.2 | 7.2 × 10−2–358.9 |
| Range of inhalation exposure to PM2.5–1 | 5.7 × 10−2–338.8 | 5.4–1011.9 |
| Range of inhalation exposure to PM10–2.5 | 0–1194.2 | 18.7–29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 874.3 874.3 |
| Range of inhalation exposure to PM20–10 | 0–25.8 | 14.5–2072.6 |
| Range of total deposited dose | 1.1 × 10−1–1334.5 | 36.5–32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 043.4 043.4 |
| Range of HA deposited dose | 5.9 × 10−2–1171.1 | 33.3–28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650.9 650.9 |
| Range of TB deposited dose | 8.1 × 10−3–62.7 | 1.2–1385.3 |
| Range of AL deposited dose | 4.5 × 10−2–100.7 | 2.0–2007.2 |
| Range of percent of HA deposited dose relative to total deposited dose | 52.4–88.4% | 84.5–93.1% |
| Range of percent of TB deposited dose relative to total deposited dose | 4.6–7.3% | 2.9–5.2% |
| Range of percent of AL deposited dose relative to total deposited dose | 7.0–40.4% | 4.1–10.3% |
In addition, for the consumer sprays, inhalation exposure of the PM10–2.5 fraction was dominant in seven out of ten products (facial nanospray, hair nanospray, skin hydrating nanomist, facial spray, hair spray, disinfectant spray, and skin hydrating mist). For the remaining three products, the PM10–2.5 inhalation exposure was either below the detection limit (silver nanospray and silver spray) or close to the detection limit (disinfectant nanospray). For powders investigated in that earlier study,23 inhalation exposure of PM10–2.5 was also the highest among the considered size fractions, but PM20–10 reached levels similar to PM10–2.5 in three out of seven powders. In addition, PM10–2.5 inhalation exposures for all powders were above the detection limit.
Another notable difference between the consumer sprays and the cosmetic powders is the release of particles in the nanosized aerosol fraction (PM0.1–0.014). This fraction was released above the detection limit levels from all tested consumer spray products. In contrast, only two out of seven cosmetic powders produced PM0.1–0.014 inhalation exposure that was detected.23 Additionally, for those products where the release of the nanosized fraction was detected, a substantially higher concentration for consumer sprays was observed compared with cosmetic powders.
Another revealing finding is that those consumer sprays that showed high abundance of nanosized particles in the TEM micrographs (silver nanospray, disinfectant nanospray and silver spray) produced the lowest inhalation total and nanosized aerosol exposures. At the same time, the two other sprays with TEM micrographs showing noticeable presence of nanostructures or nanoparticles (disinfectant spray and hair spray) produced high PM0.1–0.014 inhalation exposures. In the case of these two products, however, the TEM micrographs showed high amounts of residue, in which nanosized particles were embedded. This residue was possibly formed by organic compounds present in the products and was visible as electron-contrast plumes of undefined shape. The presence of dissolved substances that likely formed this residue may have led to generation of additional particles during product application and may have facilitated easier dispersion of primary particles. Overall, the presence of nanosized particles and structures in the original liquid products as detected by TEM did not seem to correlate with the inhalation exposure of the nanosized aerosol fraction or the larger aerosol fractions. Some spray products that showed a high number of nanosized objects in the TEM micrographs actually produced the lowest total inhalation aerosol exposures, while some produced high inhalation exposures in the nanosized fraction. Since the presence of nanosized objects in products does not seem to correlate with the concentration of airborne nanoparticles, it suggests that simply measuring the size distributions of aerosols created during the use of nanotechnology-based consumer products is not sufficient to accurately predict or assess nanomaterial exposure. A more accurate approach would be to determine the exact and relative quantities of nanomaterial(s) in a given product and then determine the masses of inhaled and deposited aerosol to determine nanomaterial inhalation exposure. At the same time, the mass fractions of nanomaterial(s) in the original product and the inhaled or deposited aerosol may differ due to 1) non-homogeneity of the product, 2) non-uniform distribution of nanomaterial(s) across different size fractions of the produced aerosol and 3) aerosol dynamics after aerosolization, particularly when a spray is not used immediately within the personal breathing zone.
As suggested earlier,16,17,23 a substantial fraction of nanomaterials is likely found in larger aerosol size fractions – albeit in an agglomerated form. The observation of a large number of nano-objects using TEM in those sprays that produced very low inhalation exposure of nanosized aerosol particles seems to support this supposition.
Another notable phenomenon observed for the consumer sprays is the absence of measurable inhalation exposure of the largest supercoarse particles (PM20–10) in all but three consumer sprays: hair nanospray, skin hydrating nanomist and hair spray. Moreover, for these three products, the measured inhalation exposures of PM20–10 were substantially lower than those of the adjacent size fraction, e.g., PM10–2.5. In the case of the cosmetic powders investigated earlier,23 the simulated use of all of the products resulted in relatively high inhalation exposures of PM20–10. One possible explanation for this phenomenon could be the difference in the mode of application of consumer sprays vs. cosmetic powders. Whereas the cosmetic powders are applied directly using a brush onto the face including the immediate vicinity of the nostrils, the consumer sprays are atomized at a distance from the nose, which leaves more time for the largest particles to settle before they could be inhaled. In addition, and probably more important, liquid atomization is a more energetic dispersion technique compared to powder application by a brush or a pad and disaggregates the material more effectively.
Looking at the deposited doses (Fig. 4 and 5), one can see that between ~52% and ~88% of all particles by mass deposited in the head airways (HA). This range indicates comparatively lower average HA deposition for the consumer sprays than for the cosmetic powders, for which the HA deposition was ~85–93%. However, among all of the investigated consumer sprays, two products with the lowest total inhalation exposure dose (silver spray and silver nanospray) also produced the lowest HA deposition fractions: 52% (regular silver) and ~64% (nano silver). For the other eight consumer sprays, the deposited dose fraction for the HA was ~85–88%. As a result of relatively low HA deposition fractions, for both the regular and nano silver sprays, high alveolar (AL) deposition fractions were computed: ~29% (nano silver) and ~40% (regular silver). In contrast, the highest AL deposited dose fraction for any of the previously investigated cosmetic powders did not exceed 10%. Hence, in the case of these two consumer sprays, both of which were observed to contain nanomaterials, deposition in the alveolar region may be more important compared to depositions in the other regions of the respiratory tract. At the same time, the total inhalation aerosol exposures for these two products were 3–5 orders of magnitude lower compared to those for other sprays, and thus one cannot conclude that the exposure of the alveolar region to particulate matter, potentially containing engineered nanomaterials, would be higher than from other sprays.
Similar to the cosmetic powders,23 the deposited dose for the tracheobronchial region was the lowest among all regions of the respiratory system for all consumer sprays, specifically ~1.5–5.6 times lower than for the alveolar region, a greater difference than for cosmetic powders (~1.5–2 times). Again, silver nanospray and silver spray had the highest difference between the TB and the AL deposited doses: a factor of ~4 and ~5.6. The factor for the other eight sprays was below 1.8 – not different from that for the cosmetic powders.
Our choice of mass as the metric for aerosol exposure when using consumer products was presented in the earlier study.23 Briefly, the number-based or surface area-based metrics would not allow adequate representation of the nanomaterial content in the total aerosols where most nano-objects exist in the form of agglomerates.23 Since all consumer sprays investigated here were in non-pressurized containers and were sprayed using a pump-based mechanism, the deagglomeration processes are not expected to have been extensive, contrary to what has been shown for the propellant-based spray products.21,32,33
The effect of particle agglomeration leads to the potential nanomaterial inhalation exposure through aerosol particle size fractions larger than 100 nm, and for some spray products, up to supercoarse particles. In addition, major differences between consumer sprays and cosmetic powders include evaporation of solvents from liquid particles after spraying and a longer residence time of aerosol particles between aerosolization and inhalation. These two phenomena provide for a possibility of a more complicated aerosol dynamics. This size distribution may greatly depend on the spray composition, e.g., water and organic solvent content, the way a product is used and the environmental conditions including humidity and temperature. The presence of solvents with molecularly dissolved substances can cause particle formation due to crystallization/solidification of these chemicals. Additionally, the dissolved chemicals can precipitate on the surface and within caverns in other particles and agglomerates influencing their size.34–38 After deposition in the respiratory system, certain chemicals can dissolve away from the deposited particles thereby altering their size and state of agglomeration,39–44 which can greatly influence nanomaterial fate in the live tissue and the resulting potential biological effects.
Compared to pure nanomaterials, the multi-ingredient nature of most nanotechnology-based consumer products (Table 1) is likely to lead to altered nanomaterial properties, including biological effects as well as different aerosol properties. These differences of nanotechnology-based products from pure nanomaterials mean that to accurately estimate potential exposure and health effects, investigation of nanotechnology-based products themselves is necessary. Investigation of pure nanomaterials that are added to nanotechnology-based consumer products should also be performed. Among the challenges warranting analysis of the actual products in addition to pure nanomaterial analysis are: (1) the difficulties in determining the presence and concentrations of engineered nanomaterials in the products, (2) the differences in aerosol production, the resulting size distributions, and its subsequent dynamics for the multi-ingredient consumer products, and (3) the effect of product matrix on surface chemistry and fate of nanomaterials deposited in the respiratory system, such as penetration of engineered nano-objects into the live tissue and the effects there, as well as their possible translocation in the body.
As with certain cosmetic powders,23 we observed nanosized particles or nanostructures in the TEM micrographs of some consumer sprays, not identified by their manufacturers as nanomaterial-containing, particularly regular silver spray and regular disinfectant spray. This again supports our argument made earlier16,17,23 that product identification and labeling as a basis for determining a consumer product's nanotechnology-based status may not adequately represent the actual nature of any given consumer product with respect to its “engineered nano status”. As our own experience of trying to analyze consumer products with respect to their content of engineered nanomaterials has shown, it may be difficult or impossible to determine the engineered nano status of any given product using current analytical techniques. For example, we could not obtain clear TEM micrographs of three out of five nanotechnology-based consumer sprays (facial nanospray, hair nanospray and skin hydrating nanomist). This was mostly due to two factors: (1) the presence of multiple ingredients in the products that obscured particulate matter and/or underwent radiolysis with volatilization under the electron beam, which may cause microscope contamination and (2) low electron contrast of particles. Due to these issues, we emphasize again the importance of accurately reporting the content of engineered nanomaterials in consumer products, so that accurate exposure assessment and health risk analysis could be performed.
Conclusions
For the consumer sprays, we observed a greater variability in the levels of inhalation exposure and deposited doses of aerosols compared with cosmetic powders, which were investigated in an earlier study. We conclude that aerosol exposure would be markedly different depending on the spray product used, which was not the case with certain cosmetic powders explored earlier.We also found that consumers would receive a measurable inhalation exposure in the nanosized aerosol fraction (PM0.1–0.014) from all consumer sprays. This is in contrast to the cosmetic powders, only two of which released detectable nanosized aerosol particles.23 This indicates that exposure to airborne nanosized particulate matter would occur from all investigated products, even from those that are not labeled as containing engineered nanomaterials. This is a very important finding showing that the release of aerosol particles <100 nm alone cannot serve as an indication of engineered nanomaterial exposure.
The inhalation exposure by mass was highest in the PM10–2.5 aerosol size fraction for the majority of the consumer sprays. At the same time, it was minimal or below the detection limit for three sprays. This high PM10–2.5 variability presented by consumer sprays was a major difference from the cosmetic powders investigated previously. If engineered nanomaterials are present in a product, they are likely distributed in all aerosol size fractions. Thus, for those products where particles in the PM10–2 size range were observed, most nanomaterials would likely be inhaled with the PM10–2.5 aerosol fraction.
We found the head airways to be the primary site of aerosol deposition with ~52–88% of all aerosol particle masses. Hence, as with the cosmetic powders, toxicological research should also focus on head airways along with the other regions of the respiratory system currently receiving more attention.
Accurate engineered nanomaterial exposure assessment can only be conducted when the quantitative content of nanomaterial(s) in the original nanotechnology-based products is known. A mandate requiring that such information from the manufacturers of nanotechnology-based consumer products be provided should be considered as a possible solution to the challenge of quantitative exposure assessment.
Declaration of interest
The authors declare no conflict of interest.Acknowledgements
This research was supported in part by the National Institute of Environmental Health Sciences (NIEHS) sponsored by the University of Medicine and Dentistry of New Jersey (UMDNJ) Center for Environmental Exposures and Disease (grant no. P30ES005022), the NSF (grant NSF-CBET-1236508), and the New Jersey Agriculture and Experiment Station (NJAES) at Rutgers University. The views expressed in this paper are solely those of the authors and do not necessarily reflect the views of the funding agencies.References
- C. E. Beaudrie, M. Kandlikar and T. Satterfield, From Cradle-to-Grave at the Nanoscale: Gaps in US Regulatory Oversight along the Nanomaterial Life Cycle, Environ. Sci. Technol., 2013, 47(11), 5524–5534 CrossRef CAS PubMed
.
- D. Park, Nanomaterials in Existing and Emerging Chemical Regulation of China, Japan, Korea, US, and the European Union, September 17th 2012. Available at SSRN: http://ssrn.com/abstract=2147746.
- R. Justo-Hanani and T. Dayan, The role of the state in regulatory policy for nanomaterials risk: Analyzing the expansion of state-centric rulemaking in EU and US chemicals policies, Research Policy, 2014, 43(1), 169–178 CrossRef PubMed
.
- S. Raj, S. Jose, U. Sumod and M. Sabitha, Nanotechnology in cosmetics: Opportunities and challenges, J. Pharm. BioAllied Sci., 2012, 4, 186 CrossRef PubMed
.
- U. Aebi, et al., Impact of Engineered Nanomaterials on Health: Considerations for Benefit-Risk Assessment, Report No. 9279204475 (European Union, 2011).
- A. D. Maynard, Nanotechnology: The Next Big Thing, or Much Ado about Nothing, Ann. Occup. Hyg., 2007, 51, 1–12 CrossRef CAS PubMed
.
- A. Bradford, R. D. Handy, J. W. Readman, A. Atfield and M. Mühling, Impact of Silver Nanoparticle Contamination on the Genetic Diversity of Natural Bacterial Assemblages in Estuarine Sediments, Environ. Sci. Technol., 2009, 43, 4530–4536 CrossRef CAS
.
- Woodrow Wilson International Center for Scholars. Project on Emerging Nanotechnologies ( 2009).
- B. Nowack,
et al., Analysis of the occupational, consumer and environmental exposure to engineered nanomaterials used in 10 technology sectors, Nanotoxicology, 2012, 7(6), 1152–1156 CrossRef PubMed
.
- F. Piccinno, F. Gottschalk, S. Seeger and B. Nowack, Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world, J. Nanopart. Res., 2012, 14, 1–11 CrossRef
.
- A. Mihranyan, N. Ferraz and M. Strømme, Current status and future prospects of nanotechnology in cosmetics, Prog. Mater. Sci., 2012, 57, 875–910 CrossRef CAS PubMed
.
- P. J. Lioy, Y. Nazarenko, T. W. Han, M. J. Lioy and G. Mainelis, Nanotechnology and Exposure Science – What Is Needed To Fill the Research and Data Gaps for Consumer Products, Int. J. Occup. Environ. Health, 2010, 16, 376–385 Search PubMed
.
- H. Hagendorfer,
et al., Size-fractionated characterization and quantification of nanoparticle release rates from a consumer spray product containing engineered nanoparticles, J. Nanopart. Res., 2010, 12, 2481–2494 CrossRef CAS
.
- K. Donaldson, X. Y. Li and W. MacNee, Ultrafine (nanometre) particle mediated lung injury, J. Aerosol Sci., 1998, 29, 553–560 CrossRef CAS
.
- T. Benn, B. Cavanagh, K. Hristovski, J. D. Posner and P. Westerhoff, The Release of Nanosilver from Consumer Products Used in the Home, J. Environ. Qual., 2010, 39, 1875–1882 CrossRef CAS
.
- Y. Nazarenko, T. W. Han, P. J. Lioy and G. Mainelis, Potential for exposure to engineered nanoparticles from nanotechnology-based consumer spray products, J. Exposure Sci. Environ. Epidemiol., 2011, 21, 515–528 CrossRef CAS PubMed
.
- Y. Nazarenko, H. Zhen, T. Han, P. J. Lioy and G. Mainelis, Potential for Inhalation Exposure to Engineered Nanoparticles from Nanotechnology-Based Cosmetic Powders, Environ. Health Perspect., 2012, 120(6), 885–892 CrossRef CAS PubMed
.
- B. Nowack and T. D. Bucheli, Occurrence, behavior and effects of nanoparticles in the environment, Environ. Pollut., 2007, 150, 5–22 CrossRef CAS PubMed
.
- I. Iavicoli, E. J. Calabrese and M. A. Nascarella, Exposure to Nanoparticles and Hormesis, Dose-Response, 2010, 8, 501–517 CrossRef CAS PubMed
.
- H. Johnston,
et al., Engineered nanomaterial risk. Lessons learnt from completed nanotoxicology studies: potential solutions to current and future challenges, Crit. Rev. Toxicol., 2013, 43, 1–20 CrossRef CAS PubMed
.
- M. E. Quadros and L. C. Marr, Silver Nanoparticles and Total Aerosols Emitted by Nanotechnology-Related Consumer Spray Products, Environ. Sci. Technol., 2011, 45, 10713–10719 CrossRef CAS PubMed
.
- M. Shimada,
et al., Development and Evaluation of an Aerosol Generation and Supplying System for Inhalation Experiments of Manufactured Nanoparticles, Environ. Sci. Technol., 2009, 43, 5529–5534 CrossRef CAS
.
- Y. Nazarenko, H. Zhen, T. Han, P. Lioy and G. Mainelis, Nanomaterial inhalation exposure from nanotechnology-based cosmetic powders: a quantitative assessment, J. Nanopart. Res., 2012, 14, 1–14 CrossRef PubMed
.
- L. Reijnders, Human health hazards of persistent inorganic and carbon nanoparticles, J. Mater. Sci., 2012, 47, 5061–5073 CrossRef CAS
.
- O. Creutzenberg, Biological interactions and toxicity of nanomaterials in the respiratory tract and various approaches of aerosol generation for toxicity testing, Arch. Toxicol., 2012, 86, 1117–1122 CrossRef CAS PubMed
.
- K. Clark,
et al., Limitations and information needs for engineered nanomaterial-specific exposure estimation and scenarios: recommendations for improved reporting practices, J. Nanopart. Res., 2012, 14, 1–14 CrossRef
.
- M. Methner, C. Beaucham, C. Crawford, L. Hodson and C. Geraci, Field Application of the Nanoparticle Emission Assessment Technique (NEAT): Task-Based Air Monitoring During the Processing of Engineered Nanomaterials (ENM) at Four Facilities, J. Occup. Environ. Hyg., 2012, 9, 543–555 CrossRef CAS PubMed
.
- G. A. Sotiriou,
et al., A novel platform for pulmonary and cardiovascular toxicological characterization of inhaled engineered nanomaterials, Nanotoxicology, 2012, 6, 680–690 CrossRef CAS PubMed
.
- P. J. Lioy,
et al., Characterization of the dust/smoke aerosol that settled east of the World Trade Center (WTC) in lower Manhattan after the collapse of the WTC September 11, 2001, Environ. Health Perspect., 2002, 110, 703–714 CrossRef CAS
.
- W. Yang, J. I. Peters and R. O. Williams III, Inhaled nanoparticles—A current review, Int. J. Pharm., 2008, 356, 239–247 CrossRef CAS PubMed
.
- R. F. Egerton, P. Li and M. Malac, Radiation damage in the TEM and SEM, Micron, 2004, 35, 399–409 CrossRef CAS PubMed
.
- C. Lorenz,
et al., Nanosized aerosols from consumer sprays: experimental analysis and exposure modeling for four commercial products, J. Nanopart. Res., 2011, 13, 3377–3391 CrossRef CAS
.
- A. W. Nørgaard,
et al., Release of VOCs and Particles During Use of Nanofilm Spray Products, Environ. Sci. Technol., 2009, 43, 7824–7830 CrossRef PubMed
.
- O. D. Velev, A. M. Lenhoff and E. W. Kaler, A Class of Microstructured Particles Through Colloidal Crystallization, Science, 2000, 287, 2240–2243 CrossRef CAS
.
- P. A. Kralchevsky and N. D. Denkov, Capillary forces and structuring in layers of colloid particles, Curr. Opin. Colloid Interface Sci., 2001, 6, 383–401 CrossRef CAS
.
- G. B. Sukhorukov,
et al., Layer-by-layer self assembly of polyelectrolytes on colloidal particles, Colloids Surf., A, 1998, 137, 253–266 CrossRef
.
- G. B. Sukhorukov,
et al., Stepwise polyelectrolyte assembly on particle surfaces: a novel approach to colloid design, Polym. Adv. Technol., 1998, 9, 759–767 CrossRef CAS
.
- F. Caruso, Nanoengineering of particle surfaces, Adv. Mater., 2001, 13, 11–22 CrossRef CAS
.
- M. B. Snipes, Long-term retention and clearance of particles inhaled by mammalian species, Crit. Rev. Toxicol., 1989, 20, 175–211 CrossRef CAS PubMed
.
- W. G. Kreyling, M. Semmler-Behnke and W. Möller, Health implications of nanoparticles, J. Nanopart. Res., 2006, 8, 543–562 CrossRef CAS
.
- K. A. Howard,
et al., RNA interference in vitro and in vivo using a chitosan/siRNA nanoparticle system, Mol. Ther., 2006, 14, 476–484 CrossRef CAS PubMed
.
- F. Petitot,
et al., Inhalation of uranium nanoparticles: Respiratory tract deposition and translocation to secondary target organs in rats, Toxicol. Lett., 2013, 217, 217–225 CrossRef CAS PubMed
.
- P. Wiebert,
et al., No significant translocation of inhaled 35-nm carbon particles to the circulation in humans, Inhalation Toxicol., 2006, 18, 741–747 CrossRef CAS PubMed
.
- P. Wiebert,
et al., Negligible clearance of ultrafine particles retained in healthy and affected human lungs, Eur. Respir. J., 2006, 28, 286–290 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3en00053b |
| ‡ Current affiliation: Department of Atmospheric and Oceanic Sciences, McGill University, 805 Sherbrooke Street West, Montreal, QC H3A 0B9, Canada. |
| This journal is © The Royal Society of Chemistry 2014 |