Selective production of hydrogen peroxide and oxidation of hydrogen sulfide in an unbiased solar photoelectrochemical cell†
Abstract
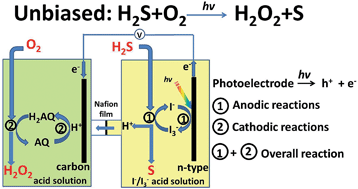
A solar-to-chemical conversion process is demonstrated using a photoelectrochemical cell without external bias for selective oxidation of hydrogen sulfide (H2S) to produce hydrogen peroxide (H2O2) and sulfur (S). The process integrates two redox couples anthraquinone/anthrahydroquinone and I−/I3−, and conceptually illustrates the remediation of a waste product for producing valuable chemicals.

 Please wait while we load your content...
Please wait while we load your content...