Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Fabricating high-energy quantum dots in ultra-thin LiFePO4 nanosheets using a multifunctional high-energy biomolecule—ATP†
Xudong
Zhang
*a,
Zhiying
Bi
a,
Wen
He
*a,
Guang
Yang
c,
Hong
Liu
d and
Yuanzheng
Yue
*ab
aInstitute of Materials Science and Engineering, Qilu University of Technology, Jinan 250353, China. E-mail: zxd1080@126.com; hewen1960@126.com; Fax: +86 531 89631080; Tel: +86 531 89631518
bSection of Chemistry, Aalborg University, DK-9000 Aalborg, Denmark. E-mail: yy@bio.aau.dk
cInternational Center for Dielectric Research, Xian Jiaotong University, Xian, 710049, China
dState Key Laboratory of Crystal Materials, Shandong University, Jinan 250100, China
First published on 11th March 2014
Abstract
By using a multifunctional high-energy biomolecule—adenosine triphosphate (ATP)—we fabricated high-energy quantum dots (HEQDs) with a feature size of less than 10 nm and used them in high-power lithium-ion batteries. We introduced high-energy phosphate bonds into the crystal structure of LiFePO4 nanoparticles and synthesized the mesoporous biocarbon nanowire coated LiFePO4 with HEQDs (MBCNW-LFP-HEQDs) by using ATP as a phosphorus source, a nucleating agent, a structural template and a biocarbon source. HEGDs were homogeneously formed inside the ultra-thin LiFePO4 nanosheet and the mesoporous biocarbon nanowire network structure was coated on the surface of the nanosheet. In LiFePO4 nanoparticles, HEQDs result in more storage sites of Li+ ions and easier transfer kinetics of electrons and lithium ions, where the kinetic transformation path between LiFePO4 and FePO4 is rather different from the path deduced from its equilibrium phase diagram. Compared to the usual LiFePO4 nanoparticle (10–100 nm) cathode, the MBCNW-LFP-HEQD cathode shows the best first discharge capacity of 197 mA h g−1 at the 0.1 C rate, which is higher than the theoretical capacity of LiFePO4 (170 mA h g−1). After 100 cycles at varied current rates: 0.1, 0.5, 1, 5 and 10 C, this cathode still delivered a high discharge capacity of 180 mA h g−1 and an ultra-high coulombic efficiency close to 100%. This is attributed to the quantum tunneling of HEQDs in LiFePO4 nanoparticles and better percolation of mesoporous biocarbon nanowire coating network structures. This work is instructive for fabrication and design of new types of electrochemical energy conversion and storage devices with extraordinary properties and functions.
Broader contextNanostructure lithiated iron phosphate (LiFePO4) has been considered as the most promising cathode candidate because of its inherent merits including low toxicity, potential for low cost, long cycle ability and high safety. Currently, one of the most popular topics is the development of controllable fabrication technology of nanosized particles. In this contribution, we report on a new technique for fabricating sub-10 nm high-energy quantum dots (HEQDs) in ultra-thin LiFePO4 nanosheets and synthesizing the mesoporous biocarbon nanowire coating LiFePO4 nanosheets with HEQDs using a high-energy biomolecule—ATP. This nanostructure provides more storage sites of Li+ ions and easier transfer kinetics of electrons and lithium ions, and displays the superior discharge capacity and an ultra-high coulombic efficiency. The fabrication strategy presented here is facile, cost-effective, and scalable, and opens up new avenues for developing optimal nanocomposite cathode materials for high performance lithium-ion batteries. |
1. Introduction
The electrode materials for the next-generation of large-scale lithium-ion batteries used for hybrid electric vehicles (HEVs) or electric vehicles (EVs) should not only fulfill the safety standard, but also possess good rate performance for both charge and discharge of batteries.1–3 In this context, nanostructured lithiated iron phosphate (LiFePO4) has been considered as the most promising cathode candidate because of its inherent merits including low toxicity, low cost, long cycle ability and high safety.4–6 However, as LiFePO4 nanoparticles have higher surface area and lower crystallinity compared to conventional LiFePO4 materials, more electrolyte is decomposed on the nanoparticle surface, leading to the enhanced formation of solid–electrolyte interfaces. This also lowers coulombic efficiency, which is one of the critical parameters for practical utilization of electrode materials, resulting in fewer storage sites of Li+ ions and sluggish kinetics of electrons and lithium ions.7,8 Recently, scientists have made progress in removing Li transport limitations in LiFePO4 through a combination of the following factors: reducing the active particle size to the nano-scale, coating active particles with carbon, incorporating dopant impurities, diluting the active mass within the electrode with the electrochemically inactive material, and coating active particles with glassy ionic conductors. Despite this progress, the innate rate capability of LiFePO4 is revealed to be still higher.9 Unfortunately, it is very difficult to achieve the theoretical capacity (170 mA h g−1) of LiFePO4 because of its intrinsic slow diffusion for Li+ and very low electronic conductivity. The specific capacity of commercially available cathode carbon-coated nanosized LiFePO4 (10–100 nm) is typically 120–160 mA h g−1. Therefore, the controllable fabrication of nanosized particles is a key for enhancing the electron transfer and lithium ion diffusion.10Defect-free crystalline LiFePO4 has an olivine structure (Pnma) and a Fe2+/3+ redox couple at 3.5 V. LiFePO4 stores Li through a two-phase transformation between FePO4 and LiFePO4 in equilibrium states. Li-ion diffusion in the lattice is uniaxial along the b-direction [010] channel via a nonlinear, curved trajectory between Li sites.11–14 G. Ceder et al.15 have demonstrated why LiFePO4 functions as a cathode at a reasonable rate. The reason is attributed to the existence of a single-phase transformation path at a very low overpotential, allowing the system to bypass nucleation and growth of a second phase. The single-phase metastable transformation path is facile in non-equilibrium states. Since lithiation in the nanoparticles occurs more homogeneously than that in the two-phase model, the stresses in the system are reduced, and thus the cycling behaviour of LiFePO4 electrodes is improved. Despite the progress, researchers still continue to seek even better electrochemical systems with new phase transformation mechanisms for the next-generation lithium-ion batteries.
Quantum dots (QDs) (<10 nm) are quasi zero dimension nanodomains containing only a small number of atoms. Owing to an extremely high surface-to-mass ratio of QDs, much more atoms are situated on the surface compared to bulk materials. The surface atoms possess higher energy of delocalized electrons and hence are kinetically more active than the interior atoms. As a consequence, QDs will have more sites bearing more lithium ions. QDs have become the center of an ever growing research effort because of their size effects, significantly enhanced kinetics, fascinating physical properties such as quantum electronic transport, quantum confinement effect, quantum tunneling effect, extremely high electron mobility, and interesting for both fundamental science and applications.16 Beyond the 10 nm technology, the quantum nature of atoms and molecules is expected to increase the transport of Li+ ions and electrons in electrodes and to harvest the extremely useful electrochemical performances as additional charge carriers via hole traps.17 HEQDs in LiFePO4 nanoparticles imply that the theoretical capacity (170 mA h g−1) of crystalline bulk LiFePO4 can be surpassed by that of QDs.18 In contrast to crystalline LiFePO4, little is known about the Li transport in QDs, but this leaves more space for further deep understanding.
In nanotechnology applications, great attention has been paid to biomaterial systems to discover new self-assembly techniques. The interaction of nanoparticles with proteins, peptides, and DNA has been studied for innovative and robust nanomaterial designs.19,20 Nanobiotechnology is one of the most powerful routes for organizing a nanoscale system with the highest accuracy. Biological systems can produce extraordinary inorganic structures and morphologies.21–24 In this work, we develop a new technique for fabricating high-energy quantum dots (HEQDs) in ultra-thin LiFePO4 nanosheets and synthesizing the mesoporous biocarbon nanowire coating LiFePO4 with HEQDs (MBCNW-LFP-HEQDs) using a high-energy biomolecule—ATP as a phosphorus source, a nucleating agent, a structural template and a biocarbon source. We demonstrate the quantum confinement effect and tuning of the electrochemical performances for breaking through theoretical capacity of LiFePO4. Electrons and Li ions can be easily transported to the HEQDs due to its tunneling barrier, which is lower than that of the usual LiFePO4 nanoparticles (10–100 nm) (ULFPNP, the ULFPNP sample is from Peking University leading Science and Technology Industry Co., Ltd.). This new technique has two important advantages: the Li transport rate in HEQDs is higher than that in ULFPNPs due to the structural disorder; and the electrode capacity of HEQDs is larger than the theoretical capacity of bulk crystalline LiFePO4. Besides the obvious huge increase in capability value and coulombic efficiency of LiFePO4, they can be synthesized more easily and with low cost; they can self-assemble and, most importantly, they can display new electrochemical performances and behaviors. Such a simple and scalable approach may also be applied to other cathode systems, boosting up the capacity for various Li batteries.
2. Experimental
Synthesis of MBCNW-LFP-HEQDs
The reagents used in this work include FeCl3 (99%, Tianjin Kermel Chemical Reagent Co., Ltd.), acyl nucleoside triphosphate disodium salt (Na2ATP, 10 mg ml−1, Xuzhou Ryen Pharma. Co., Ltd.), HCl (36–38%, Laiyang Economic and Technological Development Zone Fine Chemical Plant), ascorbic acid (99.5%, Tianjin Bodi Chemical Co., Ltd.), and Li2CO3 (97%, Tianjin Damao Chemical Co., Ltd.). Distilled water was used during synthesis of the samples.MBCNW-LFP-HEQDs were prepared using the biotemplate and carbon thermal reduction method. The general synthesis procedure is described as follows. Na2ATP in a stoichiometric ratio was added into a beaker and incubated for 30 min at different temperatures (30, 40 and 50 °C) in a water bath pot. Then the Na2ATP was immediately cooled with liquid nitrogen to release the surface charge of anionic phosphate groups in the ATP biomolecule, and thus the Na2ATP biotemplate solution were obtained. FeCl3 was dissolved in distilled water and added to the Na2ATP biotemplate solution with magnetic stirring at room temperature, and then adjusted pH to 3 with HCl. A yellow solution with the FePO4 sediment was formed. Finally, the mixture solutions were stirred vigorously for 12 hours. The FePO4 sediment was dried in an oven at 120 °C for constant weight, and then the dried FePO4 mixture was washed and centrifuged two times with distilled water to remove the soluble sodium ions, and then dried to a constant weight in an oven at 120 °C again. The dry FePO4–ATP mixtures were mixed with Li2CO3 and ascorbic acid in the stoichiometric ratio—nFe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nLi
nLi![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nacid = 1
nacid = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for 6 h through a dry ball-milling process. The resulting mixtures were annealed at 300 °C for 3 h, and then sintered at different temperatures (700, 750 and 800 °C) in a N2 atmosphere for 8 h. The heating rate was kept relatively low, i.e., 5 °C min−1, to avoid structural collapse due to fast crystallization. The final product obtained was in the form of black powder, and the samples were marked as MBCNW-LFP-HEQD-1 (with the ATP biotemplate incubated at 30 °C), MBCNW-LFP-HEQD-2 (with the ATP biotemplate incubated at 40 °C), MBCNW-LFP-HEQD-3 (with the ATP biotemplate incubated at 50 °C).
1 for 6 h through a dry ball-milling process. The resulting mixtures were annealed at 300 °C for 3 h, and then sintered at different temperatures (700, 750 and 800 °C) in a N2 atmosphere for 8 h. The heating rate was kept relatively low, i.e., 5 °C min−1, to avoid structural collapse due to fast crystallization. The final product obtained was in the form of black powder, and the samples were marked as MBCNW-LFP-HEQD-1 (with the ATP biotemplate incubated at 30 °C), MBCNW-LFP-HEQD-2 (with the ATP biotemplate incubated at 40 °C), MBCNW-LFP-HEQD-3 (with the ATP biotemplate incubated at 50 °C).
Characterization
The morphology, porous structure and crystal structure of the synthesized samples were characterized using a high-resolution transmission electron microscope (HRTEM) and an atomic force microscope (AFM). HRTEM images were obtained using a Philips Tecnai 20U-TWIN microscope, working at 300 kV. A trace amount of sample was dispersed in ethanol solution by sonication, and then deposited on a carbon-coated copper grid. HRTEM images were obtained at the Scherzer defocus (Δf = −41.25 nm) to optimize the transfer function of the optical system balancing the effect of spherical aberration. AFM measurements were carried out using an atomic force microscope (IIIa AFM), and the samples were prepared by adding the ethanol suspension with the dispersed particles dropwise onto a freshly cleaved mica piece. Energy-dispersive spectrometer (EDS) measurements were performed using a JEM-ARM200F scanning transmission electron microscope (STEM). X-ray photoelectron spectroscopy (XPS) measurements were performed using a Kratos Axis Ultra DLD (delay line detector) spectrometer equipped with a monochromatic Al Kα X-ray source (1486.6 eV). The phase composition of the synthesized samples were analyzed by X-ray diffraction (XRD) employing a Cu-Kα X-ray diffractometer (PANalytical X'Pert PRO; Netherlands). The diffraction patterns were collected over a diffraction angle 2θ range of 5–90°, with an acquisition time of 12 s at 0.02° step size. The N2 adsorption–desorption isotherms and Barrett–Joyner–Halenda pore size distributions were carried out at 77 K using a automatic surface area analyzer (Micromeritics, Gemini V2380, USA) under continuous adsorption conditions. Fourier transform infrared spectroscopy (FT-IR) measurements were performed using a Nicolet Nexus spectrometer (Nicolet, NEXUS 470, USA) and by using a KBr wafer technique. The carbon content in the samples was determined using a Vario EL IIICHN elemental analyzer. The fluorescence of the sample was probed directly using a ECLIPSE80i Fluorescent microscope (Nikon, Japan).Electrochemical evaluation
The charge–discharge performances were determined with CR 2032 coin cells. The cathode materials were prepared by mixing the synthesized sample with acetylene black and polyvinylidene fluoride (PVDF) in a weight ratio of 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 in N-methyl pyrrolidone to ensure homogeneity. Then the mixture was pressed to a piece on an Al-foil with about 0.02 mm in thickness, dried under an air atmosphere at 60 °C for 5 h and a vacuum atmosphere at 120 °C for 8 h and cut into circular strips of 15 mm in diameter. The mass calculation of active materials was carried out based on the 85% of difference between the mass of synthesized materials coated Al foil and the mass of pristine Al foil. The cells were assembled in a glove box filled with high-purity argon, where lithium metal was used as an anode, a polypropylene film as the separator, and 1 M LiPF6 in an electrolyte consisting of ethylene carbonate–dimethyl carbonate–ethylene methyl carbonate in a volume ratio of 1
10 in N-methyl pyrrolidone to ensure homogeneity. Then the mixture was pressed to a piece on an Al-foil with about 0.02 mm in thickness, dried under an air atmosphere at 60 °C for 5 h and a vacuum atmosphere at 120 °C for 8 h and cut into circular strips of 15 mm in diameter. The mass calculation of active materials was carried out based on the 85% of difference between the mass of synthesized materials coated Al foil and the mass of pristine Al foil. The cells were assembled in a glove box filled with high-purity argon, where lithium metal was used as an anode, a polypropylene film as the separator, and 1 M LiPF6 in an electrolyte consisting of ethylene carbonate–dimethyl carbonate–ethylene methyl carbonate in a volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The charge–discharge performances of the synthesized samples were tested using a Channels battery analyzer (CT3008W) at different current densities between 2.5 and 4.2 V cut-off voltages using the coin-cells. The electrochemical impedance (EIS) and cyclic voltammetry (CV) measurements were performed with a PARSTAT 2263 electrochemical workstation. EIS was also recorded with the frequency ranging from 100 kHz to 10 mHz and a AC signal of 5 mV in amplitude as the perturbation. The voltage range of the CV measurements was 2.5–4.2 V and the scanning rate was from 0.1 to 1 mV s−1. All the tests were performed at room temperature.
1. The charge–discharge performances of the synthesized samples were tested using a Channels battery analyzer (CT3008W) at different current densities between 2.5 and 4.2 V cut-off voltages using the coin-cells. The electrochemical impedance (EIS) and cyclic voltammetry (CV) measurements were performed with a PARSTAT 2263 electrochemical workstation. EIS was also recorded with the frequency ranging from 100 kHz to 10 mHz and a AC signal of 5 mV in amplitude as the perturbation. The voltage range of the CV measurements was 2.5–4.2 V and the scanning rate was from 0.1 to 1 mV s−1. All the tests were performed at room temperature.
3. Results and discussion
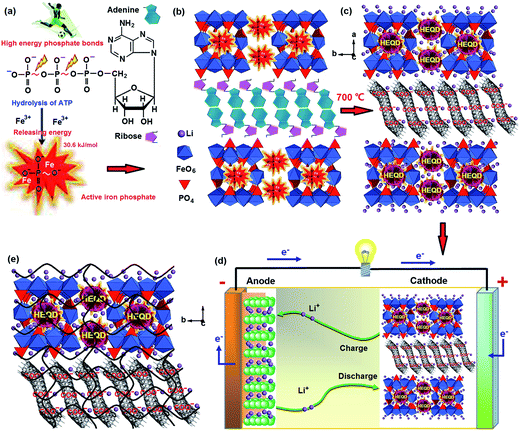
ATP-enhanced electrochemical performances of the nanostructured LiFePO4 were first demonstrated. A schematic diagram of a biological method is summarized in Fig. 1. Adenosine triphosphate (ATP) is the high-energy biomolecule and its critical part is the phosphorus part—the triphosphate. There are high-energy phosphorus bonds in the triphosphate and they release a large amount of free energy upon the reaction,25,26 which can be used as a phosphorus source and a nucleating agent during the synthesis. The structure of ATP has an ordered carbon compound backbone, which can be used as a structural template and a biocarbon source. Three phosphorus groups are connected by oxygen ions, and each of these oxygens has a negative charge, causing repulsion force among these oxygens, i.e., increasing the potential energy (Fig. 1a). So the ATP exhibits increased interactions with Fe cations and can serve as a nucleating agent for active iron phosphate growth. During incubation with the iron chloride precursor, all ATP biomolecules were loaded with iron ions to synthesize active FePO4 and a complex structure was formed by the self-assembly of iron ions and ATP biomolecules (Fig. 1b). During pyrolytic degradation, the carbon compound backbone of ATP is decomposed to form a mesoporous biocarbon nanowire network, while an external or internal self-created atmosphere can prevent Fe2+ from oxidation and effectively restricts the growth of the LiFePO4 nanoparticles (Fig. 1c). The high-energy phosphorus bonds form HEQDs in LiFePO4 nanoparticles. In the synthetic process, the chemical bond linkages between ATP biomolecules with iron ions and the in situ nanocomposite linkages between LiFePO4 nanosheets with the mesoporous biocarbon nanowire network were studied by FT-IR (see Fig. S2d†). The results indicate that hydroxyl (OH−) in the ribose and OPO32− radicals in the triphosphate part play the important role in the chemical interaction between the ATP biomolecules and the iron ions. The decrease of the P–O bond length shows that the high-energy phosphate bonds have been introduced into the crystal structure of LiFePO4 nanoparticles (Table S2j†). There are in situ nanocomposite linkages between the LiFePO4 nanoparticles and the mesoporous biocarbon nanowire coating network. The X-ray diffraction patterns of the synthesized samples (Fig. S2a and b†) show only the peaks indexed as LiFePO4. Through the XRD and FT-IR spectroscopy results, we have verified that the deposition and self-assembly mechanism shown in Fig. 1 is feasible for synthesizing MBCNW-LFP-HEQDs.The synthesized material was assembled as a cathode in a lithium-ion battery using lithium metal foil as a negative electrode (Fig. 1d). The results obtained by battery tests show that the sample has good reversibility, excellent discharge capacity and ultra-high coulombic efficiency. The total channel length between surfaces of HEQDs is very small (<5 nm) (Fig. 2a), and all sites are available by rapid diffusion through unblocked channels. Li+ ions can circumvent HEQDs by migrating between different channels. The HEQDs offer such an opportunity for Li+ to cross channels (Fig. 1e).
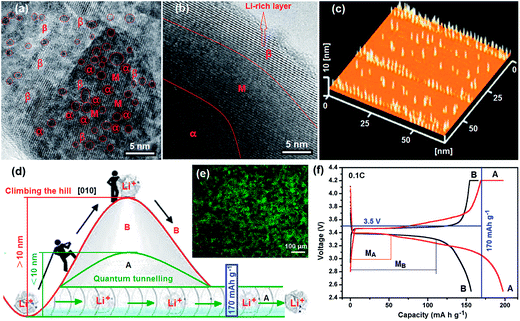
HRTEM is a powerful tool to study properties of materials on the atomic scale that allows for direct imaging of the atomic structure of the sample. HRTEM image formation relies on phase contrast. The phase contrast imaging technique is very sensitive to the sample thickness. The interaction of the electron wave with the crystallographic structure of the sample is complex and in this case the image cannot be interpreted in terms of crystal structure projection, but a qualitative idea of the interaction can readily be obtained.27 In order to probe the fine nanostructure of LiFePO4, HRTEM analysis was employed and the images were recorded at a negative Scherzer defocus. Fig. 2a and b show HRTEM images of a LiFePO4 nanosheet with HEQDs and a normal crystalline LiFePO4 nanoparticle without HEQDs (ULFPNP), respectively. According to the image contrast mechanism in the Weak Phase Object (WPO) approximation,28 the ultra-thin LiFePO4 nanosheet shown in Fig. 2a and the ultra-thin edge of the ULFPNP shown in Fig. 2b can be identified as the WPO, and at the negative Scherzer defocus Li atomic columns in the β Li-rich phase (LiβFePO4) are imaged as bright spots. It is found that Fig. 2a shows a brighter background than that shown in Fig. 2b, implying that the particle shown in Fig. 2a is thinner. Fig. 2a shows that the HEQDs with an average size of about 3 nm (see the dark spots enclosed by the red rings) are embedded in an ultra-thin LiFePO4 nanosheet, and that there are distinct lattice dislocations and various antisite defects in the HEQD nanocrystal domains. The HEQDs appear as dark spots because the HEQD has a higher atomic density. The existence of HEQDs in the LiFePO4 structure was also confirmed by the AFM image (Fig. 2c) and the fluorescent micrograph (Fig. 2e) of the MBCNW-LFP-HEQD-2 sample. Fig. 2c shows the size and height distributions of HEQDs embedded in a ultra-thin LiFePO4 nanosheet, where the standing yellow rods represent HEQDs with a height of 10 nm. Fig. 2e exhibits green fluorescence at the excitation wavelength of 480 nm. However, there is no dark spot shown in Fig. 2b, only showing regular lattice fringes of LiFePO4. The area between the darker α Li-poor phase (LiαFePO4) and the brighter β Li-rich phase (LiβFePO4) is a miscibility gap marked as M.29 The mobility limitation of the two-phase boundary of FePO4–LiFePO4 formed owing to lithium extraction from LiFePO4 could be diminished in a solid solution, LixFePO4.30 However, the M of the LiαFePO4–LiβFePO4 binary phase has almost vanished as shown in Fig. 2a.31 We found that the average number, size and distribution of HEQDs in the LiFePO4 nanosheet can be controlled by changing the incubating temperature of ATP and the growth time during the synthesized process (ESI Fig. S1†), thus providing a way of controlling the growth of HEQDs for different applications.
Ceder et al.32 demonstrated that most channels in small enough nanoparticles are unblocked, making all sites accessible by very rapid migration of Li+ through the channels. The QDs can not only store quickly more Li ions because of the higher specific surface area, but also increase the Li+ conductivity and the electrochemical reaction centers, and also reduce the effective diffusion length of Li+ ions because of the small size of channels. Especially, since the interfaces among QDs account for several tens of percentage by volume, the interfacial effect is dominant in overall lithium transport. The interfaces of QDs act as fast pathways for Li+ ions and provide more active sites for Li+ ions in LiFePO4, and hence, enhance the lithium ion intercalation and deintercalation, leading to an extra storage of Li+ and a high capacity.33,34 Therefore the excessive lithium storage could explain why the theoretical capacity value of LiFePO4 is surpassed. QDs alter the LiFePO4 phase transition pathways during the electrochemical process. To demonstrate this effect, the first discharge and charge profiles of the MBCNW-LFP-HEQD-2 cathode (A) and the ULFPNP cathode (B) at the 0.1 C rate using CR 2032 coin cells are compared and analyzed (Fig. 2f, AB). Unfortunately, in the ULFPNP structure, even the lowest energy path, which occurs by Li migrating to the nearest Li channel through a vacant intermediate Fe2+ site, has an activation barrier much higher than that within a certain channel (Fig. 2d, B). So it is very difficult to achieve the theoretical capacity of LiFePO4 because of fewer storage sites of Li+ ions and sluggish kinetics of electrons and lithium ions in the ULFPNP structure (Fig. 2f, B). Based on the understanding of anti-site defects, the HEQD is a promising way to contribute to high electronic conductivity. Two-dimensional diffusion in the LiFePO4 nanosheet with HEQDs is significantly different from one-dimensional diffusion in ULFPNPs. The presence of HEQDs has a drastic effect on the rate, at which Li ions can move through the LiFePO4 crystal via quantum tunnelling (Fig. 2d, A) and can make Li channels cross-over possible (Fig. 1e). Therefore, the first discharge capacity of the MBCNW-LFP-HEQD-2 cathode can break through the theoretical capacity of LiFePO4 and delivered the best high first discharge capacity of 197 mA h g−1 (116% of the theoretical value) at the 0.1 C rate (Fig. 2f, A). Comparing with the miscibility gap MB of the ULFPNP cathode (Fig. 2f, B), HEQDs in a single LiFePO4 nanosheet are in an instability state due to increased interface energy, resulting in a decrease of the miscibility gap MA (Fig. 2f, A) of the MBCNW-LFP-HEQD cathode and a increase of the solid solution region of LiFePO4.35 Compared with other material design as we published previously,21,22 although the electrochemical properties of synthesized cathodes were enhanced and the cathode delivered the best high first discharge capacity of 160.4 mA h g−1 at the 0.1 C rate, it also is very difficult to achieve the theoretical capacity of LiFePO4. Based on the above analysis, it is clear that Li ions in the MBCNW-LFP-HEQD cathode can migrate more quickly along the Li-ion channels, and this may improve the ionic properties of LiFePO4.
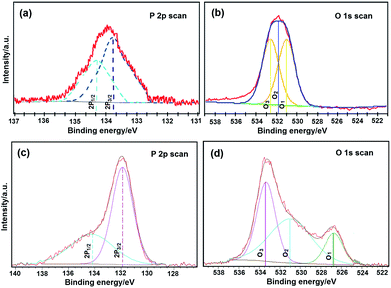
To investigate the role of high-energy phosphate bonds in the crystal structure of LiFePO4 nanoparticles, high-resolution X-ray photoelectron spectroscopy (HRXPS) was applied. Fig. 3 shows phosphorus 2p (P 2p) and oxygen 1s (O 1s) HRXPS curves from the ULFPNP and MBCNW-LFP-HEQD-2 samples, respectively. In P 2p HRXPS, the spectra were fitted to the P 2p doublet, the 2p1/2 peak and the 2p3/2 peak, which is split by 0.5 eV. The higher energy, the lower intensity peak is the 2p1/2 peak, and another is the 2p3/2 peak. They show two different chemical states for phosphorus. In O 1s HRXPS, the spectra were fitted to the O 1s three peaks, O1, O2 and O3. They show three different chemical states for oxygen. The O1 species at the low binding energy belong to O2˙ ions, whereas O2 species with a medium binding energy are attributed to O˙ and O2˙ ions in the oxygen deficient regions mainly caused by oxygen vacancies, VO. The high binding energy species O3 belong to the absorbed or dissociated oxygen or OH species on the surface of the sample.36 The fitted comparison data of P 2p and O 1s peaks for ULFPNP and MBCNW-LFP-HEQD-2 samples are presented in Table S1.† In Table S1,† it is found that the binding energy at the 2p3/2 peak and the O1 1s peak from MBCNW-LFP-HEQD-2 is much lower than those of ULFPNPs and the feature of their O 1s HRXPS is different, revealing that there are high-energy phosphate bonds in the crystal structure of MBCNW-LFP-HEQD-2. Besides, the percentage of O2 species in ULFPNP and MBCNW-LFP-HEQD-2 samples were 15.2% and 22.6%, respectively. Therefore, the MBCNW-LFP-HEQD-2 sample has more oxygen vacancies and higher activity, which are associated with active sites for high-energy phosphate bonds and could contribute to higher rate performance. This is consistent with the HRTEM results (see Fig. 4f).
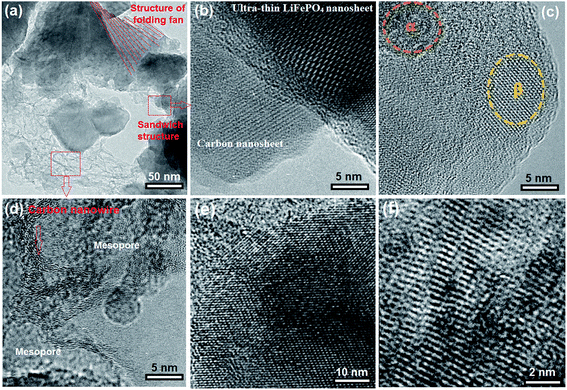
To understand the effect of ATP biotemplates on the structure of synthesis materials, we characterized the synthesized samples using various methods. Fig. 4 shows HRTEM images of the MBCNW-LFP-HEQD-2 sample. In Fig. 4a it is seen that the MBCNW-LFP-HEQD-2 sample has the layer structures with folding fan and sandwich. LiFePO4 nanosheets were tethered to biocarbon nanosheets mainly through the mesoporous biocarbon nanowire attachment. This indicates the precipitation reactions of LiFePO4 nanoparticles occur on the ATP biomolecules. The brightest area shown in Fig. 4a corresponds to monolayers, whereas the darker area is composed of more layers of nanosheets. Fig. 4b shows an ultra-thin LiFePO4 nanosheet and a biocarbon nanosheet. Fig. 4c exhibits the HEQDs with the α-Li-deficient phase (LiαFePO4) and the β-Li-rich phase (LiβFePO4) in an ultra-thin LiFePO4 nanosheet. Fig. 4d shows the biocarbon nanowire structures. The highly conductive mesoporous biocarbon nanowire wrapping around the LiFePO4 nanosheet assists the electron migration during the charge/discharge processes, diminishing the irreversible capacity at the first cycle and leading to higher coulombic efficiency without fading at various C-rates. Effective electron transport is another important requirement for kinetics, since insertion/extraction of Li ions must be accompanied by electrons transfer to keep the charge balance. Fig. 4e shows a lattice image of the ultra-thin LiFePO4 nanosheet, exhibiting the partial densities of states of Fe, P, O and Li atoms in the LiFePO4 nanosheet structure with the stoichiometry and Li/Fe exchange-defects. In other words, it can be clearly seen that LiFePO4 with the Li/Fe exchange defect has more electron states, which is another favorable condition to achieve higher electronic conductivity.
Anyway, the special electronic structure ensures that the material with the Li/Fe exchange defect may exhibit a higher discharge capacity (Fig. 2f, A). Controlling the concentration, type and segregation of the native defects via tuning the synthesis conditions is an instructional way to enhance the electrical conductivity of LiFePO4. It should be mentioned that anti-site defects sometimes offer an opportunity for Li ions to cross-over between channels. The antisite defect in the LiFePO4 structure is a kind of intrinsic defect, which is easily formed during the synthesis process. They are characterized by an exchange of Li and Fe in the lattice. Henkelman et al.37 reported that a concerted Li-ion diffusion mechanism around the anti-site defect in FePO4 is found to have a low barrier of 0.35 eV, allowing for facile cross-channel diffusion at room temperature. Using HRTEM, as shown in Fig. 4f, we have observed possible point defects in the fine microstructure of the MBCNW-LFP-HEQD-2 sample, involving combinations of Li vacancies and interstitials, Li+ on Fe2+ sites, Fe2+ on Li+ sites, Fe3+ on Fe2+ sites, oxygen and Fe2+ vacancies.38 The most of HEQDs embedded in the LiFePO4 nanosheet seem the nearest neighbor antisite defects and α-Li-poor phases (LiαFePO4) (Fig. 2a) and they have the lowest formation energy of about 0.515–0.550 eV.39
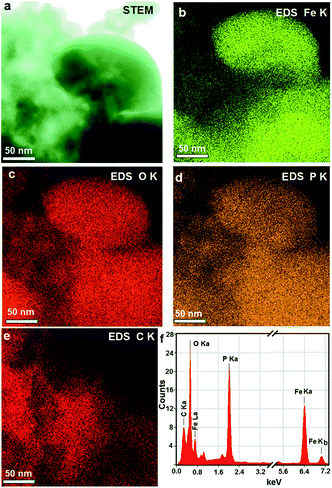
Besides, we characterized the elemental distribution mappings of the MBCNW-LFP-HEQD-2 sample at the nanometer level using scanning transmission electron microscopy (STEM) and X-ray energy-dispersive spectrometry (EDS) (Fig. 5). Fig. 5a shows a bright field STEM image of the MBCNW-LFP-HEQD-2 sample. High resolution STEM-EDS analyses show the distributions of Fe, P, O and C of the MBCNW-LFP-HEQD-2 sample in the nanoscale range, respectively (Fig. 5b–e), where Fe, P and O elements are evenly distributed in the nanometer range and C elements are distributed on the surface particles. Fig. 5f shows the EDS spectra of the MBCNW-LFP-HEQD-2 sample, revealing that the sample contains Fe, P, O and C elements, and the atomic ratio of Fe, P, O and C is also close to the stoichiometric proportion of initial reactants. The crystal structure and the mesoporous structure of the different synthetic samples and the ULFPNP sample were evaluated and compared by X-ray diffraction (XRD) and N2 adsorption–desorption methods (Fig. S2†). The results show that all of samples synthesized under different conditions have an orthorhombic olivine-type structure of pure phase LiFePO4 and the amorphous residual carbon (Fig. S2a†). However, the MBCNW-LFP-HEQD-2 sample possesses better layered structures (Fig. S2b†), better mesoporous structures (Fig. S2c and S3†) and higher crystallinity (Table S3†) through biomineralization processes. It is likely that the high-energy phosphate groups with negative charges in high-energy biomolecule ATP are not only the groups preferentially bonded with iron ions, but also the most important contributor to the free energy barrier for iron phosphate biomineralization at room temperature and pressure.23 The summary of the structural features and performances for the MBCNW-LFP-HEQDs samples and the ULFPNP sample is given in Tables S2 and S3 in the ESI S3.†
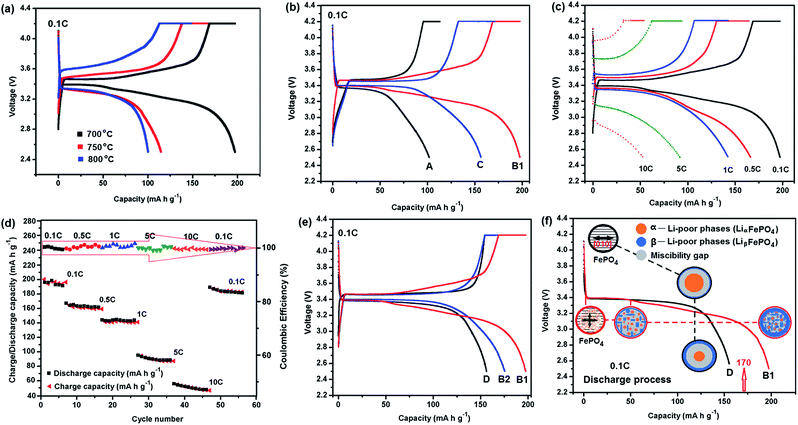
The electrochemical performances of the different MBCNW-LFP-HEQD cathodes were evaluated and compared by charge–discharge tests, electrochemical impedance spectroscopy (EIS) and cyclic voltammogram (CV) methods using CR 2032 coin cells. Fig. 6a and b show the first charge–discharge curves of the different cathodes synthesized at different temperatures at 0.1 C, respectively. The MBCNW-LFP-HEQD-2 cathode synthesized at 700 °C delivered a discharge capacity of 197 mA h g−1 at a low discharge rate of 0.1 C, which is higher than the theoretical capacity of LiFePO4 (170 mA h g−1). After 56 cycles, the cell still delivered a ultra-high discharge capacity of 180 mA h g−1 and retained 91.4% of its initial capacity at the 0.1 C rate (Fig. 6e, B2). To the best of our knowledge, this is the best high discharge capacity performance for the LiFePO4 cathode material reported so far and even better than those of previously reported LiFePO4/C composites.40–42
One potential issue of using hybrid materials as electrodes is stability. We tested at different rates and much higher number of cycles for the MBCNW-LFP-HEQD-2 cathode and compared with the existing ULFPNP cathode to more comprehensively evaluate its performance. Although the specific capacity gradually decreases with the increasing current rate (Fig. 6c), a high coulombic efficiency very close to 100% has been achieved at different rates. Even at the high current rate of 10 C, the cell also maintained high coulombic efficiency of 99%. Fig. 6d shows the cyclability of the MBCNW-LFP-HEQD-2 cathode at different rates on which the charge and discharge capacity curves are almost overlapping due to the ultrahigh coulombic efficiency (as shown as the red arrows in the inset). We also compared the rate capability of the MBCNW-LFP-HEQD-2 cathode before and after cycling at different rates. The results are shown in Fig. 6e. The rate capability of the MBCNW-LFP-HEQD-2 cathode after 56 cycles at varied current rates: 0.1, 0.5, 1, 5, and 10 C is 180 mA h g−1. It should be noticed that as long as the current rate reverses back to low current rate, the cell capacity can still be higher than the theoretical capacity of LiFePO4. The original capacity was recovered, confirming structural stability and indicating that the integrity of the MBCNW-LFP-HEQD-2 cathode material has been maintained even after high rate charge and discharge. Fig. S6 in the ESI S2† shows that the MBCNW-LFP-HEQD-2 cathode still retained 91.4% of its initial capacity at the 1 C rate after 100 cycles. These clearly demonstrate that our nanostructure architecture of LiFePO4 with HEQDs is tolerant to varied charge and discharge currents and much higher number of cycles compared with existing electrode materials.
Fig. 6f shows schematic diagrams of kinetic transformation paths between LiFePO4 and FePO4 in the first discharge process of MBCNW-LFP-HEQD-2 (B1) and ULFPNP (D) cathodes at 0.1 C. For the ULFPNP sample, the miscibility gap MB region is greater than 10 nm (Fig. 2b), resulting in the discharge platform MB shown in Fig. 2f increases (see the black dotted line path shown in Fig. 6f). In the LiFePO4 nanosheet structure of the MBCNW-LFP-HEQD-2 sample, it is found that the miscibility gap MA region was decreased to less than 5 nm by embedding HEQDs (Fig. 2a). This means that solid solution behavior is exhibited in a bigger and more homogenous range in the LiFePO4 structure. So the changed kinetic transformation paths between LiFePO4 and FePO4 in the first discharge process of the MBCNW-LFP-HEQD-2 cathode were changed (see the red dotted line path shown in Fig. 6f), and makes the coexistence of two phases in a single particle unstable. The smaller discharge platform MA shown in Fig. 2f means that LiFePO4 nanosheets have a short path for lithium intercalation and de-intercalation, also showing the main reason why the specific capacity exceeds the theoretical value at low rate.
The reason why the specific capacity of the synthetic electrode gradually decreases with the increasing current rate could be attributed to many factors. The change of the nanostructure and the decrease of its active surface area for the positive electrode during cycling as well as the increased electrode resistance are suggested to be partly responsibly for reducing the electrochemical reaction rate and capacity. Besides the cathode material itself, other possible factors could be influential, e.g., degradation of the lithium metal foil anode, growth of the passivated surface film layer on the electrodes, decomposition of the binder and the electrolyte, and corrosion of both the current collector and the separator. All these factors will affect the charge transfer kinetics.43 To better characterize the capacity fading mechanisms with increasing current rate, the electrochemical impedance spectroscopy (EIS) and cyclic voltammogram (CV) performances of the synthetic electrodes are measured as shown in Fig. S4 and S5,† respectively. The diffusion coefficient value DLi of lithium ions is calculated in Table S2c,† which can help to clarify the ohmic resistance and polarization resistance behaviors coincident with capacity loss. The results of EIS and CV tests indicate that the MBCNW-LFP-HEQD-2 electrode has the lower charge transfer resistance and the outstanding reversibility of the lithium extraction/insertion reactions. The electrochemical kinetics of the MBCNW-LFP-HEQD-2 electrode is easier regarding Li+ extraction than that of the ULFPNP blank electrode and has the better rate performance (please see ESI, Fig. S6†). This is attributed to the excellent nanocomposite of HEQDs embedded inside the crystal structure and mesoporous biocarbon nanowire network coating in situ on the surface of LiFePO4 nanosheets.
4. Conclusions
In summary, we have developed the LiFePO4/C nanocomposite by using a multifunctional high-energy biomolecule—ATP, in which HEQDs are embedded in ultra-thin LiFePO4 nanosheet particles and mesoporous biocarbon nanowire interconnected networks were coated on the surface of the nanosheet. By studying the synthesis mechanism we demonstrate that the ATP biomolecules as multifunctional biotemplates are able to control the formation of ultra-thin LiFePO4 nanosheet particles and to direct the HEQDs to be embedded inside the nanosheet structure, and to introduce high-energy phosphate bonds into the crystal structure of LiFePO4 nanoparticles simultaneously. The mesoporous nanoarchitecture ensures not only intimate contact between the liquid electrolyte and active LiFePO4 nanoparticles, but also high electronic conductivity for both facile mass transfer and facile charge transfer. At the low current rate, MBCNW-LFP-HEQD cathodes deliver a much higher specific capacity than the theoretical value and a ultrahigh coulombic efficiency. This nanocomposite structure provides high electronic conductivity for enhancing the rate capability of lithium insertion reaction, and scaling up can be readily achieved. Through biomimetic material design, the LiFePO4 cathode material can display electrochemical performances and behaviors for nanoelectronics functionalities.Although MBCNW-LFP-HEQD nanocomposites have been successfully synthesized and the contributions of HEQDs and mesoporous biocarbon nanowire network structures to LiFePO4 in improving the electrochemical performance have been discussed, some fundamental questions still remain and need to be investigated in detail. An in-depth study of the surface chemistry of MBCNW-LFP-HEQD nanocomposites will direct the optimization of the nature of the nanocomposites. A high tap density of MBCNW-LFP-HEQD nanocomposites is considered to be an important factor for practical applications. An effective route to improve the material's tap density is to design novel structures, including spherical, aggregated and especially microspherical structures composed of tightly compacted nanocrystallites.5 We have synthesized the cage-like LiFePO4 shell@biocarbon core microspheres with higher discharge energy density at higher current rates by using the same approach combined with hydrothermal treatments. This novel method might become a very competitive candidate for future high energy density C/LiFePO4 production.
Acknowledgements
The authors thank Natural Science Foundation of China (Grant no. 51172132, 51272144, 51042003, 50925205 and 51372142) for the financial support; they also thank the Taishan scholarship program in the field of Glass and Ceramics for the technological support.Notes and references
- A. Manthiram, A. Vadivel Murugan, A. Sarkar and T. Muraliganth, Energy Environ. Sci., 2008, 1, 621 CAS.
- K. T. Lee and J. Cho, Nano Today, 2011, 6, 28 CrossRef CAS PubMed.
- Y. G. Wang, P. He and H. S. Zhou, Energy Environ. Sci., 2011, 4, 805 CAS.
- K. T. Lee, W. H. Kan and L. F. Nazar, J. Am. Chem. Soc., 2009, 131, 6044 CrossRef CAS PubMed.
- J. Wang and X. Sun, Energy Environ. Sci., 2012, 5, 5163 CAS.
- L. X. Yuan, Z. H. Wang, W. X. Zhang, X. L. Hu, J. T. Chen, Y. H. Huang and J. B. Goodenough, Energy Environ. Sci., 2011, 4, 269 CAS.
- S. Y. Chung, J. T. Bloking and Y. M. Chiang, Nat. Mater., 2002, 1, 123 CrossRef CAS PubMed.
- Y. J. Lee, H. Yi, W. J. Kim, K. Kang, D. S. Yun, M. S. Strano, G. Ceder and A. M. Belcher, Science, 2009, 324, 1051 CrossRef CAS PubMed.
- C. Delacourt, P. Poizot, J. M. Tarascon and C. Masquelier, Nat. Mater., 2005, 4, 254 CrossRef CAS.
- L. H. Hu, F. Y. Wu, C. T. Lin, A. N. Khlobystov and L. J. Li, Nat. Commun., 2013, 4, 1687 CrossRef PubMed.
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930 CrossRef CAS PubMed.
- A. Yamada, H. Koizumi, S. I. Nishimura, N. Sonoyama, R. Kanno, M. Yonemura and Y. Kobayashi, Nat. Mater., 2006, 5, 357 CrossRef CAS PubMed.
- B. Kang and G. Ceder, Nature, 2009, 458, 190 CrossRef CAS PubMed.
- S. I. Nishimura, G. Kobayashi, K. Ohoyama, R. Kanno, M. Yashima and A. Yamada, Nat. Mater., 2008, 7, 707 CrossRef CAS PubMed.
- R. Malik, F. Zhou and G. Ceder, Nat. Mater., 2011, 10, 587 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, M. I. Katsnelson, I. V. Grigorieva, S. V. Dubonos and A. A. Firsov, Nature, 2005, 438, 197 CrossRef CAS PubMed.
- F. Zhou, T. Maxisch and G. Ceder, Phys. Rev. Lett., 2006, 97, 155704 CrossRef.
- Y. B. Zhang, Y. W. Tan, H. L. Stormer and P. Kim, Nature, 2005, 438, 201 CrossRef CAS PubMed.
- O. E. Semonin, J. M. Luther, S. Choi, H. Y. Chen, J. B. Gao, A. J. Nozik and M. C. Beard, Science, 2011, 334, 1530 CrossRef CAS PubMed.
- U. O. S. Seker, T. Ozel and H. V. Demir, Nano Lett., 2011, 11, 1530 CrossRef CAS PubMed.
- X. G. Zhang, X. D. Zhang, W. He, Y. Z. Yue, H. Liu and J. Y. Ma, Chem. Commun., 2012, 48, 10093 RSC.
- X. D. Zhang, W. He, Y. Z. Yue, R. M. Wang, J. X. Shen, S. J. Liu, J. Y. Ma, M. Li and F. X. Xu, J. Mater. Chem., 2012, 22, 19948 RSC.
- X. Y. Du, W. He, X. D. Zhang, Y. Z. Yue, H. Liu, X. G. Zhang, D. D. Min, X. X. Ge and Y. Du, J. Mater. Chem., 2012, 22, 5960 RSC.
- M. Jensen, R. Keding, T. Hoche and Y. Z. Yue, J. Am. Chem. Soc., 2009, 131, 2717 CrossRef CAS PubMed.
- F. Borel, R. Q. Han, A. Visser, H. Petry, S. J. H. van Deventer, P. L. M. Jansen and P. Konstantinova, Hepatology, 2012, 55, 821 CrossRef CAS PubMed.
- S. J. Ferguson, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 16755 CrossRef CAS PubMed.
- E. Abe, Chem. Soc. Rev., 2012, 41, 6787 RSC.
- D. B. Williams and C. B. Carter, Transmission Electron Microscopy: A Textbook For Materials Science, Springer, New York, NY, USA, 1996 Search PubMed.
- G. Kobayashi, S. I. Nishimura, M. S. Park, R. Kanno, M. Yashima, T. Ida and A. Yamada, Adv. Funct. Mater., 2009, 19, 395 CrossRef CAS.
- B. Ellis, L. K. Perry, D. H. Ryan and L. F. Nazar, J. Am. Chem. Soc., 2006, 128, 11416 CrossRef CAS PubMed.
- P. Gibot, M. Casas-Cabanas, L. Laffont, S. Levasseur, P. Carlach, S. Hamelet, J. M. Tarascon and C. Masquelier, Nat. Mater., 2008, 7, 741 CrossRef CAS PubMed.
- R. Malik, D. Burch, M. Bazant and G. Ceder, Nano Lett., 2010, 10, 4123 CrossRef CAS PubMed.
- J. Maier, Nature, 2005, 4, 805 CrossRef CAS.
- Q. J. Xiang, J. G. Yu and M. Jaroniec, J. Am. Chem. Soc., 2012, 134, 6575 CrossRef CAS PubMed.
- J. Maier, Nat. Mater., 2005, 4, 805 CrossRef CAS.
- Z. S. Wu, L. L. Xue, W. C. Ren, F. Li, L. Wen and H. M. Cheng, Adv. Funct. Mater., 2012, 22, 3290 CrossRef CAS.
- G. K. P. Dathar, D. Sheppard, K. J. Stevenson and G. Henkelman, Chem. Mater., 2011, 23, 4032 CrossRef CAS.
- G. R. Gardiner and M. S. Islam, Chem. Mater., 2010, 22, 1242 CrossRef CAS.
- C. A. J. Fisher, V. M. H. Prieto and M. S. Islam, Chem. Mater., 2008, 20, 5907 CrossRef CAS.
- G. X. Wang, H. Liu, J. Liu, S. Z. Qiao, G. Q. Max Lu, P. Munroe and H. Ahn, Adv. Mater., 2010, 22, 4944 CrossRef CAS PubMed.
- C. M. Doherty, R. A. Caruso, B. M. Smarsly, P. Adelhelm and C. J. Drummond, Chem. Mater., 2009, 21, 5300 CrossRef CAS.
- X. L. Wu, L. Y. Jiang, F. F. Cao, Y. G. Guo and L. J. Wan, Adv. Mater., 2009, 21, 2710 CrossRef CAS.
- M. K. Mahapatra and R. J. Bodnar, Appl. Phys. A: Mater. Sci. Process., 2009, 95, 493 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ee44187c |
| This journal is © The Royal Society of Chemistry 2014 |