New insights into the chemistry of di- and trimetallic iron dithiolene derivatives. Structural, Mössbauer, magnetic, electrochemical and theoretical studies†‡
Sonia
Bruña
a,
Isabel
Cuadrado
a,
Esther
Delgado
*a,
Carlos J.
Gómez-García
b,
Diego
Hernández
a,
Elisa
Hernández
a,
Rosa
Llusar
c,
Avelino
Martín
d,
Nieves
Menéndez
e,
Victor
Polo
fg and
Félix
Zamora
a
aDepartamento de Química Inorgánica, Universidad Autónoma de Madrid, 28049 Madrid, Spain. E-mail: esther.delgado@uam.es
bInstituto de Ciencia Molecular, 46980 Paterna, Valencia, Spain
cDepartamento de Química Física y Analítica, Universidad de Castellón, 12071 Castellón de la Plana, Spain
dDepartamento de Química Orgánica y Química Inorgánica, Universidad de Alcalá de Henares, 28871, Alcalá de Henares, Spain
eDepartamento de Química Física Aplicada, Universidad Autónoma de Madrid, 28049 Madrid, Spain
fDepartamento de Química Física Universidad de Zaragoza e Instituto de Biocomputación y Física de Sistemas Complejos (BIFI), 50009 Zaragoza, Spain
gCSIC, Insituto de Química Física Rocasolano, 28006, Madrid, Spain
First published on 1st July 2014
Abstract
Reaction of Fe3(CO)12 with 1,2-dithiolene HSC6H2Cl2SH affords a mixture of complexes [Fe2(CO)6(μ-SC6H2Cl2S)] 1, [Fe2(SC6H2Cl2S)4] 2 and [Fe3(CO)7(μ3-SC6H2Cl2S)2] 3. In the course of the reaction the trimetallic cluster 3 is first formed and then converted into the known dinuclear compound 1 to afford finally the neutral diiron tetrakis(dithiolato) derivative 2. Compounds 2 and 3 have been studied by Mössbauer spectroscopy, X-ray crystallography and theoretical calculations. In compound 2 the metal atoms are in an intermediate-spin FeIII state (SFe = 3/2) and each metal is bonded to a bridging dithiolene ligand and a non-bridging thienyl radical (S = 1/2). Magnetic measurements show a strong antiferromagnetic coupling in complex 2. Cyclic voltammetry experiments show that the mixed valence trinuclear cluster 3 undergoes a fully reversible one electron reduction. Additionally, compound 3 behaves as an electrocatalyst in the reduction process of protons to hydrogen.
Introduction
Transition metal complexes with 1,2-dithiolenes are a subject of high research interest as a consequence of their rich redox chemistry, versatility in the coordination modes, biological function, as well as their well-known magnetic and electrical conducting properties.1 Ott et al.2 have described the formation of the di-iron derivatives [Fe2(CO)6(μ-SRS)] (R = C6H4 or C6H2Cl2) as the sole compound from the thermal reaction of Fe3(CO)12 with the corresponding dithiolene. On the other hand, Huttner et al.3 have reported analogous reactions but using aliphatic dithiols, such as HSCH2CH2SH or HSCH2CH2CH2SH, in which a trinuclear cluster [Fe3(CO)7(μ-SRS)2] is isolated together with the dinuclear species [Fe2(CO)6(μ-SRS)]. To our knowledge these two triiron clusters and the analogous complex [Ru3(CO)7(μ-SCH2CH2S)2]4 are the only examples reported on mixed valence heptacarbonyl trinuclear derivatives of group 8 containing dithiolato ligands. Additionally, the closely related compound [Fe3(CO)7(μ3-SC6H4N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)2] containing a diazenylbenzenethiolato group is also known.5
N)2] containing a diazenylbenzenethiolato group is also known.5
Compounds [Fe2(CO)4L2(μ-SRS)] (L = CO, phosphine or CN−; R = aliphatic or aromatic groups) are being studied as valuable models of the active site of the [FeFe] hydrogenase.6 Additionally, the role of the mixed valence clusters [Fe3(CO)7(μ-SCH2CH2S)2]7 and [Fe4{MeC(CH2S)3}2(CO)8]8 as electrocatalysts for the reduction of the protons to hydrogen has also been explored.
We have recently obtained a one dimensional coordination polymer of an iron dithiolene derivative, {[K2(μ-H2O)2(thf)4][Fe2(SC6H2Cl2S)4]}n, with rather unexpected physical properties: (i) it is the first coordination polymer containing an “s” group metal as a bridging building block showing electrical conductivity; (ii) it represents the first example of a coordination polymer showing two electrical transition phases; and (iii) it shows electrical bi-stability.9 This coordination polymer is isolated from the reaction of [Fe2(CO)6(μ-SC6H2Cl2S)] with HSC6H2Cl2SH, in the presence of K2CO3. These interesting results have prompted us to extend our work to evaluate this reaction under similar experimental conditions but in the absence of K2CO3. This slight synthetic change has led to the formation of the neutral diironIII,III tetrakis(dithiolato) derivative [Fe2(SC6H2Cl2S)4] 2. In addition, the synthesis of the mixed valence cluster [Fe3(CO)7(μ3-SC6H2Cl2S)2] 3 is also described. The redox properties of [Fe3(CO)7(μ3-SC6H2Cl2S)2] 3 have been examined by electrochemical techniques.
Results and discussion
Synthesis and characterization of complexes 2 and 3
A toluene solution of compound [Fe2(CO)6(μ-SC6H2Cl2S)] 1 and the 1,2-dithiolene HSC6H2Cl2SH in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio was refluxed under stirring for 24 h (Scheme 1). The solid formed was filtered and crystallized in acetone/n-hexane (1
3 ratio was refluxed under stirring for 24 h (Scheme 1). The solid formed was filtered and crystallized in acetone/n-hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at −20 °C to yield the purple compound 2.
2) at −20 °C to yield the purple compound 2.
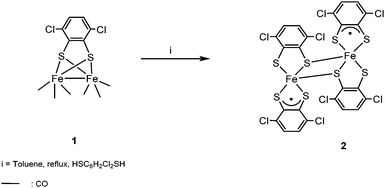
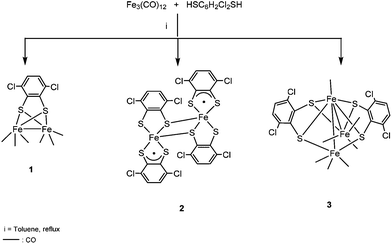
The microanalytical data and the presence of the parent ion in the mass spectrum suggested the formulation of complex 2 as [Fe2(SC6H2Cl2S)4]. The molecular structure of this neutral compound was confirmed by single crystal X-ray diffraction. The metal oxidation states were assigned based on Mössbauer studies and DFT calculations (vide infra). The high insolubility of compound 2 in the most common organic solvents, together with the experimental observation that a dark residue was always retained on the top of the column chromatograph used to purify compound 1, obtained from Fe3(CO)12 with HSC6H2Cl2SH,2 prompted us to reinvestigate whether compound 2 is also formed in this reaction. Thus, after keeping the mixture stirred under refluxing toluene for 45 min, the solvent was removed and the residue was purified on silica gel. A carefully performed column chromatography allowed us to separate three bands: [Fe2(CO)6(μ-SC6H2Cl2S)] 1, and two new bands corresponding to [Fe2(SC6H2Cl2S)4] 2 and [Fe3(CO)7(μ3-SC6H2Cl2S)2] 3 (Scheme 2).
The structure of the mixed valence cluster 3 was confirmed by single crystal X-ray diffraction. We have observed that longer reaction times and larger amounts of dithiolene, in comparison with the procedure described before, improve the yields of the new derivatives 2 (17.3% yield) and 3 (6.4% yield) (see the Experimental section). We have also studied the reaction between Fe3(CO)12 and HSC6H2Cl2SH in the presence of ONMe3. In this case, a remarkable increase in the yield of compound [Fe2(SC6H2Cl2S)4] 2 (40% yield) is observed while only trace amounts of [Fe3(CO)7(μ3-SC6H2Cl2S)2] 3 were obtained.
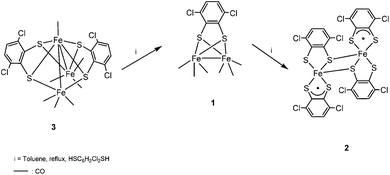
In order to know whether there is any relationship between compounds 1–3, a mixture of 3 and HSC6H2Cl2SH was left in refluxing toluene for 3 h. Afterwards, compound 1 was isolated as the major product together with traces of 2. This result in addition to the conversion of the dinuclear carbonyl derivative 1 in the neutral compound 2, as commented before, suggests that the cluster [Fe3(CO)7(μ3-SC6H2Cl2S)2] 3 is the first compound formed in the reaction of Fe3(CO)12 with HSC6H2Cl2SH. This process implies the substitution of CO groups by dithiolato ligands and the cleavage of one Fe–Fe bond in the precursor Fe3(CO)12. In a second step, probably the rupture of Fe–Fe and Fe–S bonds as well as formation of a new Fe–Fe bond in 3 would yield compound [Fe2(CO)6(μ-SC6H2Cl2S)] 1 and, finally, total substitution of carbonyls by dithiolato in compound 1 generates [Fe2(SC6H2Cl2S)4] 2 (Scheme 3).
The IR spectrum of compound 3 exhibits νCO bands at 2077 (m), 2054 (vs), 2022 (w), 2016 (s), 1999 (vw) and 1977 (s) cm−1 corresponding to terminal carbonyl ligands. The pattern of the IR spectrum of 3 is rather similar to that reported for [Fe3(CO)7(μ-SRS)2] (R = CH2CH2 and CH2CH2CH2).3 In the latter compounds an additional band corresponding to the presence of a semi-bridging CO ligand was also observed but this feature is not observed in the spectrum of 3. Moreover, a resonance at δ 7.10 ppm, for the protons assigned to the C6H2Cl2 groups is shown in its 1H NMR spectrum. Finally, the mass spectrum of 3 shows the parent ion and other ions assigned to the successive loss of CO groups.
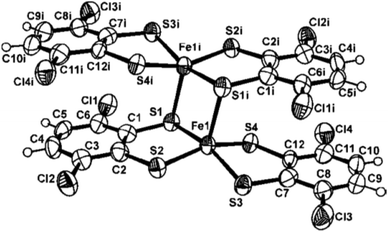
X-ray diffraction, Mössbauer, magnetic and theoretical studies of compound 2
The molecular structure of complex 2 has been determined by the neutral diferric complex. Selected bond distances and angles are given in the figure caption (Fig. 1). The [Fe(SC6H2Cl2S)2] moiety in complex 2 presents an iron centre which is S,S-bonded to one dithiolato ligand and one thienyl ligand. Additionally, each FeL2 entity acts as μ-sulfur bridging two metal centres leading to a distorted 4 + 1 square based pyramidal geometry around the iron atoms.Certainly, from ligand bond distance analysis there is some indication that the terminal non-bridging dithiolato ligands are in their oxidized form. An average of 1.758(2) Å for the [S(4)–C(12)] and [S(3)–C(7)] distances as well as a bond length of 1.43(1) Å for C(7)–C(12) are observed for this terminal non-bridging thienyl ligand, whereas the shortest C–C bond length of 1.40(1) Å [C(1)–C(2)] and an average of 1.77(2) Å for the S–C bond distances are found for the terminal bridging dithiolato group. These data agree with those previously reported species [FeIII2{S2C2(C6H4-p-CH3)2}4]10 and [FeIII2{S2C2(C6H4-p-OCH3)2}4].11 To our knowledge, the two former compounds and [Fe2(SC6H2Cl2S)4] 2 are the only examples on neutral FeIIIFeIII tetrakis(dithiolato) dimers whose crystal structures have been reported.
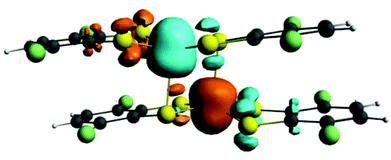
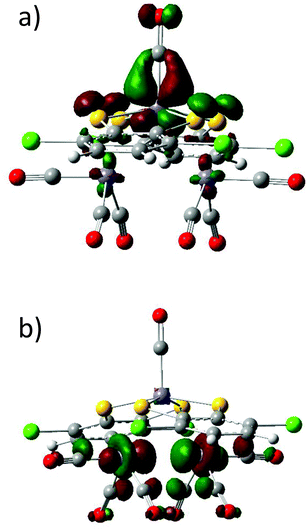
Previous studies carried out on various members of the electron transfer series [Fe2(dithiolene)4]n (n = from +1 to −2) combining DFT calculations with Mössbauer data support a +III oxidation state on both metal atoms independently of the dimer charge.1i,10 To confirm this assignment in the case of compound 2, its electronic structure was investigated by means of DFT calculations using the ADF program.12 Calculations were performed on the experimental geometry (ESI). The spin density plot for complex 2, represented in Fig. 2, shows three α and three β spins mainly located at the iron atoms. Two other unpaired electrons with α and β spins are respectively located on the two non-bridging terminal ligands and they are distributed among the sulfur, S3 and S4, and carbon, C1 and C2, atoms giving support to the radical character (S = 1/2) of the non-bridging ligands as inferred from the experimental bond lengths. The spin density for compound 2 constitutes the signature of an antiferromagnetic state.
To confirm theoretical predictions the magnetic properties of complex 2 were investigated.
The product of the magnetic susceptibility and the temperature, χmT for compound 2 shows a room temperature value of ca. 1.6 emu K mol−1 and a continuous decrease to reach a value of ca. 0.15 emu K mol−1 at 2 K (Fig. 3). This behaviour indicates that compound 2 presents a strong antiferromagnetic coupling that is expected to arise from the coupling of the two FeIII ions and the two S = 1/2 radicals. If we assume that the thienyl ligand is the terminal one, we obtain the magnetic exchange scheme depicted in the insets in Fig. 3. Since the FeIII atoms in dithiolene dimeric complexes can present a S = 3/2 or a S = 1/2 spin ground state,9 we have tested both possibilities (ESI) in our fitting procedure using the package MAGPACK.13,14 The model using a S = 3/2 ground spin state for the FeIII ions reproduces very satisfactorily the magnetic properties of compound 2 with the following parameters: g = 2.0 (fixed value), J1 = −228 cm−1, J2 = −686 cm−1, a temperature independent paramagnetism (Nα) of 3.0 × 10−4 emu mol−1 and a monomeric FeIII paramagnetic impurity of 1.0% (solid line in Fig. 3). To reduce the number of adjustable parameters we have fixed the g value to 2.0. This fit shows that the strongest AF interaction occurs between the FeIII ions and the thienyl radicals through the direct double sulfur bridge (J2) and that the Fe–Fe interaction through the double S bridge also leads to a strong AF coupling (J1 = −228 cm−1), in the range of those observed in other similar FeIII-dithiolate dimers.9 This result confirms the oxidation state III assumed for the Fe atoms in 2. Note that the very strong J2 coupling indicates the presence of a strong orbital overlap between the FeIII ion and the S atoms of the thienyl radical, in agreement with the planarity of the thienyl radical and the relatively small deviation of the FeIII atom from the basal plane. These two strong couplings give rise to the spin orientation indicated in the inset in Fig. 3, where the S = 3/2 spins are antiparallel as well as the two S = 1/2 of the thienyl radicals. This spin distribution leads to a total S = 0 ground spin state for the FeIII dimer, in agreement with the magnetic properties and with the theoretical predictions.
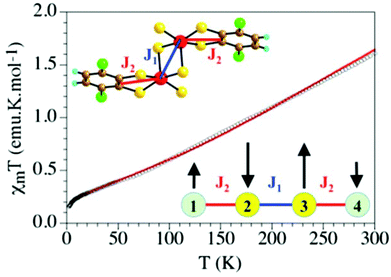 | ||
| Fig. 3 Thermal variation of the χmT product for compound 2. Solid line is the best fit to the model. | ||
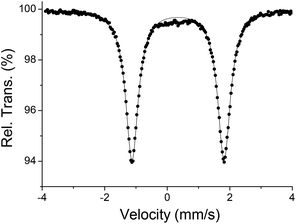
Mössbauer studies also confirm the +III oxidation state assignment for the metal atoms. Thus, the zero-field 57Fe Mössbauer spectrum of complex 2 recorded at 77 K (Fig. 4) shows a single doublet with isomer shift, δ = 0.345(2) mm s−1, and quadrupole splitting, |ΔEQ| = 2.94(1) mm s−1, in agreement with theoretical calculation (δ = 0.378 mm s−1, and |ΔEQ| = 2.853 mm s−1) as well as the reported values for other FeIII square-pyramidal complexes.1i,11
Finally, we have to indicate that we were unable to study the electrochemical behaviour of compound 2 due to its insolubility in CH2Cl2 and instability in CH3CN and thf solutions.
X-ray diffraction, Mössbauer and theoretical studies of compound 3
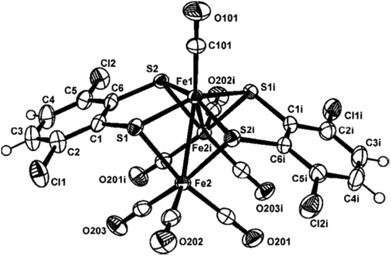
The molecular structure of compound 3 is displayed in Fig. 5. Selected bond distances and angles are listed in the figure caption (Fig. 5). Taking into account the 18 electron rule compound [Fe3(CO)7(μ3-SC6H2Cl2S)2] 3 is an electron precise 50 e− cluster.The central heptacoordinated Fe(1) iron atom exhibits a distorted capped trigonal prism geometry, while the two external Fe(2) iron atoms are coordinated to the central Fe(1) atom, three carbonyl ligands and two sulfur atoms to complete a distorted octahedral geometry. A C2 axis through the Fe1–C101–O101 unit makes equal both Fe(1)–Fe(2) bond distances, with a value of 2.715(2) Å. This interatomic distance is consistent with the presence of a metal–metal bond. However, a non-bonding distance of 3.22(1) Å is observed between Fe(2)⋯Fe(2i) centres. The three metal Fe atoms form a bent triangular cluster with a Fe(2)–Fe(1)–Fe(2i) bond angle of 72.71(5)°. This feature contrasts with the Fe(2)–Fe(1)–Fe(2) angle of 178.7(1)° found in the linear compound [Fe3(CO)7(μ3-SC6H4N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)2]5 and the reported values of 151.8(1)° and 156.22(4)° for the quasi-linear clusters [Fe3(CO)7(μ-SCH2CH2S)2]3,7 (compound 4) and [Fe3(CO)7(μ-SCH2CH2CH2S)2],15 respectively. The internal iron atom is linked to a terminal CO ligand [O(101)–C(101)–Fe(1) angle of 180.0(4)°]. This is in contrast with the semi-bridging coordination mode observed for the CO ligand in the related [Fe3(CO)7(SRS)2 (R
N)2]5 and the reported values of 151.8(1)° and 156.22(4)° for the quasi-linear clusters [Fe3(CO)7(μ-SCH2CH2S)2]3,7 (compound 4) and [Fe3(CO)7(μ-SCH2CH2CH2S)2],15 respectively. The internal iron atom is linked to a terminal CO ligand [O(101)–C(101)–Fe(1) angle of 180.0(4)°]. This is in contrast with the semi-bridging coordination mode observed for the CO ligand in the related [Fe3(CO)7(SRS)2 (R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2CH23,7 and CH2CH2CH215)] compounds. Additionally, in compound 3 each dithiolato group acts as a triple bridging ligand. Although two isomers (syn and anti) could be expected depending upon the orientation of the dithiolato groups, only the syn isomer has been observed for 3. In contrast, the anti-isomer is observed in compounds [Fe3(CO)7(μ-SCH2CH2S)2],3,7 [Fe3(CO)7(μ-SCH2CH2CH2S)2]15 and [Fe3(CO)7(μ3-SC6H4N
CH2CH23,7 and CH2CH2CH215)] compounds. Additionally, in compound 3 each dithiolato group acts as a triple bridging ligand. Although two isomers (syn and anti) could be expected depending upon the orientation of the dithiolato groups, only the syn isomer has been observed for 3. In contrast, the anti-isomer is observed in compounds [Fe3(CO)7(μ-SCH2CH2S)2],3,7 [Fe3(CO)7(μ-SCH2CH2CH2S)2]15 and [Fe3(CO)7(μ3-SC6H4N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)2],5 while in the case of the analogous ruthenium cluster [Ru3(CO)7(μ-SCH2CH2S)2] both isomers have been isolated, although the anti-isomer is the most abundant.4 Slight differences are observed in the mean central Fe–S and outer Fe–S bond distances in compounds [Fe3(CO)7(μ-SCH2CH2S)2] 43,7 and [Fe3(CO)7(μ-SCH2CH2CH2S)2].15 However, the four bond distances between the sulfur atoms and the internal iron atom [S(1)–Fe(1) of 2.208(2) Å and S(2)–Fe(1) of 2.196(1) Å] are shorter than the corresponding bond lengths between the sulfur and the external iron atoms [S(1)–Fe(2) of 2.305(2) Å and S(2)–Fe(2i) of 2.310(2) Å]. These differences can be attributed to the triple bridging mode of the dithiolene ligand in 3 which forces a bent Fe–Fe–Fe structure and a lengthening of the Fe–Fe bond.
N)2],5 while in the case of the analogous ruthenium cluster [Ru3(CO)7(μ-SCH2CH2S)2] both isomers have been isolated, although the anti-isomer is the most abundant.4 Slight differences are observed in the mean central Fe–S and outer Fe–S bond distances in compounds [Fe3(CO)7(μ-SCH2CH2S)2] 43,7 and [Fe3(CO)7(μ-SCH2CH2CH2S)2].15 However, the four bond distances between the sulfur atoms and the internal iron atom [S(1)–Fe(1) of 2.208(2) Å and S(2)–Fe(1) of 2.196(1) Å] are shorter than the corresponding bond lengths between the sulfur and the external iron atoms [S(1)–Fe(2) of 2.305(2) Å and S(2)–Fe(2i) of 2.310(2) Å]. These differences can be attributed to the triple bridging mode of the dithiolene ligand in 3 which forces a bent Fe–Fe–Fe structure and a lengthening of the Fe–Fe bond.
As far as we know, the crystal structures of compounds [Fe3(CO)7(μ-SCH2CH2S)2],3,7 [Fe3(CO)7(μ-SCH2CH2CH2S)2],15 [Ru3(CO)7(μ-SCH2CH2S)2],4 [Mn3(CO)6(μ-SCH2CH2CH2S)3]16 and [Os3(CO)10(μ-SCH2CH2S)]17 are the only examples reported on transition metal carbonyl trinuclear clusters, containing dithiolato ligands.
It is noteworthy that compound 3 exhibits some remarkable differences with those clusters: (i) it represents the first example of this type of clusters containing aromatic instead of aliphatic dithiolato ligands and (ii) a triple bridging coordination mode is observed for the –SC6H2Cl2S– ligand, while the aliphatic dithiolato groups bridge two metals in the precedent examples.
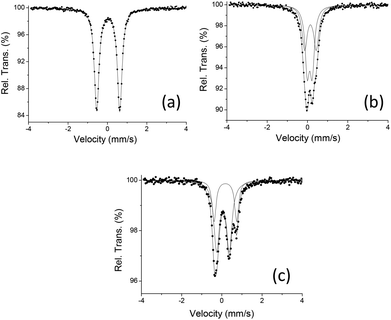
We have prepared the reported trinuclear cluster [Fe3(CO)7(μ-SCH2CH2S)2] 43,7 in order to carry out comparative studies with the new cluster [Fe3(CO)7(μ3-SC6H2Cl2S)2] 3. Additionally, they have been extended to the dinuclear derivative [Fe2(CO)6(μ-SC6H2Cl2S)] 1.2 DFT studies of the three compounds have been carried out at the OPBE level.18 These complexes possess a singlet ground state in agreement with its diamagnetic nature. The experimental geometries are well reproduced by gas-phase geometry optimizations (Tables S1, S2 and Fig. S1‡). In agreement with the statement before commented, the metal–metal distance in 3 is ca. 0.2 and 0.15 Å longer than those in compounds 1 and 4, respectively. On the other hand, the optimized Fe(1)–S bond distances in compound 3 are ca. 0.15 Å shorter than the distances between the external Fe(2) atoms and their bonded sulfur atoms and the same tendency is also calculated for compound 4. Although a mixed valence FeIFeIIFeI state has been reported for [Fe3(CO)7(SRS)2 (R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2CH23,7 and CH2CH2CH215)] compounds in which each dithiolato is bridging two iron metals, the presence of two triple bridging “SC6H2Cl2S” groups in cluster 3 may also yield another mixed valence possibility such as FeIIFe0FeII. In order to gain insight a Mössbauer experiment has been carried out on compound 3. The complexity associated with the isomer shift-oxidation state correlation in organometallic complexes, that can be explained on back-donation introduced by certain ligands which increase the electron density at the nuclei,19,20 prompted us to extend the spectroscopic study to compounds 1 and 4 so that similar coordination environments could be compared (Fig. 6, Table 1).
CH2CH23,7 and CH2CH2CH215)] compounds in which each dithiolato is bridging two iron metals, the presence of two triple bridging “SC6H2Cl2S” groups in cluster 3 may also yield another mixed valence possibility such as FeIIFe0FeII. In order to gain insight a Mössbauer experiment has been carried out on compound 3. The complexity associated with the isomer shift-oxidation state correlation in organometallic complexes, that can be explained on back-donation introduced by certain ligands which increase the electron density at the nuclei,19,20 prompted us to extend the spectroscopic study to compounds 1 and 4 so that similar coordination environments could be compared (Fig. 6, Table 1).
| Compound | T (K) | δ (mm s−1) | |ΔEQ| (mm s−1) | I |
|---|---|---|---|---|
| a IS isomer shift relative to metallic α-Fe at room temperature (mm s−1). b Using the OPBE/TZP method. | ||||
| 1 | 295 | −0.040 (2) | 1.128(4) | 100% |
| 77 | 0.058(1) | 1.170 (1) | 100% | |
| 1 | 0 | 0.130 | 1.042 | 100% |
| 3 | 295 | 0.041(2) | 0.261(7) | 60% |
| 0.072 (3) | 0.59(1) | 40% | ||
| 77 | 0.116(1) | 0.265(4) | 65% | |
| 0.156 (2) | 0.593(8) | 35% | ||
| 3 | 0 | 0.182 | 0.215 | 66% |
| 0.240 | 0.614 | 33% | ||
| 4 | 295 | −0.010(6) | 0.614(9) | 66% |
| 0.100(7) | 1.12(1) | 33% | ||
| 77 | 0.046(1) | 0.624(2) | 63% | |
| 0.176(2) | 1.112(2) | 37% | ||
| 4 | 0 | 0.117 | 0.640 | 66% |
| 0.274 | 1.047 | 33% | ||
The spectrum of compound 3 (Fig. 6b) results from the convolution of two quadrupole doublets, one with an area almost twice the other confirming the presence of two of the three iron atoms in a similar environment indicating mixed valence states. Although a similar spectrum has been recorded for cluster [Fe3(CO)7(μ-SCH2CH2S)2] 4, different isomer shift values have been found for both clusters (Fig. 6c, Table 1). On the other hand, as seen in Fig. 6a, compound [Fe2(CO)6(μ-SC6H2Cl2S)] 1 shows a single quadrupole doublet with an isomer shift value of δ = 0.058 mm s−1 at 77 K that is similar to that found at the same temperature for the outer iron atoms in compound [Fe3(CO)7(μ-SCH2CH2S)2] 4 (δ = 0.046 mm s−1). These data are consistent with a +I oxidation state,6d,19 while values of δ = 0.116(1) and 0.156(2) mm s−1 were found for cluster [Fe3(CO)7(μ3-SC6H2Cl2S)2] 3. This fact could indicate that compound 3 shows different iron oxidation states to those reported for 4. On the other hand, analogous behaviour has been reported for the FeIFeI compound [Fe2{μ-SCH2C(CH3)2CH2S}(PMe3)2(CO)4] (δ = 0.06 mm s−1) and its oxidised species FeIFeII [Fe2(μ-CO){μ-SCH2C(CH3)2CH2S}(PMe3)2(CO)3]PF6 (δ = 0.105 and 0.190 mm s−1).20 Therefore, the above mentioned data do not allow us to assign the formal oxidation state, FeIFeIIFeI or FeIIFe0FeII, for compound 3.
Mössbauer experimental parameters have been calculated for compounds 1, 3 and 4 starting from their computed electronic structures and the results are listed in Table 1 together with the experimental values. The OPBE/TZP energies, <S2> values and electron density at the Fe nucleus (ρ(0)) employed for the calculation of the Mössbauer isomer shift are given in ESI (Table S3‡). A good agreement between the experimental and calculated data is observed.
Electrochemistry of compound 3
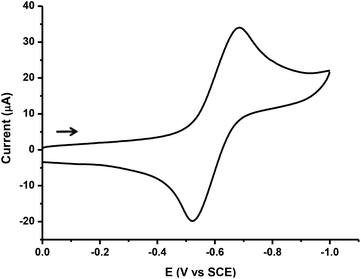
The electrochemical behaviour of the trimetallic cluster [Fe3(CO)7(μ3-SC6H2Cl2S)2] 3 was examined by cyclic voltammetry (CV) in dichloromethane using [n-Bu4N][PF6], as a supporting electrolyte.When the potential is scanned in the cathodic direction, from 0 to −2.0 V region, cluster 3 shows a reduction process at Epc = −0.69 V vs. SCE (Fig. 7).
For this redox couple, the plot of peak current (ip) versus v1/2 was linear, indicating that the redox process was diffusion controlled. Likewise, the voltammetric features (ipc/ipa essentially equal to unity, ΔEp values about 85–100 mV at slow scan rates, and Ep independent of v) show that the reduction of compound 3 is chemically reversible on the voltammetric time scale.21 The number of electrons transferred in the redox process was estimated by cyclic voltammetry, by comparison of the current intensity of the CV of 3 with that of an equimolar amount of decamethylferrocene (Cp*2Fe)22 (Fig. S2‡). This established that the cathodic couple of 3 involves the transfer of one electron, and suggests the formation of the monoanionic species [Fe3(CO)7(μ3-SC6H2Cl2S)2]−. A simplistic analysis of the frontier orbitals in 3 (Fig. 8) suggests that the electron enters a LUMO orbital which is basically formed by the dz2 orbitals of the external iron atoms. This fact could indicate that the higher oxidation state is located on them.
In contrast, the reduction process reported for complex 4 is irreversible and shows a cathodic shift of ca. 300 mV with regard to that of compound 3.7 A frontier orbital analysis on 4 (Fig. S3‡) indicates that upon reduction the entering electron occupies a Fe–Fe antibonding orbital. For comparison, the redox behaviour of the related di-iron compound [Fe2(CO)6(μ-S2C6H2Cl2)] 1 has been investigated (Fig. S4‡). This bimetallic compound undergoes a single reversible diffusion-controlled reduction at −0.90 V vs. SCE, which also shows a cathodic shift with respect to the first reduction peak of 3 (−0.69 V). Consequently, the reduction of trimetallic compound 3 is thermodynamically easier than that of compounds 1 and 4.
In the anodic region, tri-iron cluster 3 exhibits a quasi-reversible oxidation wave (Fig. S5‡), whose current intensity closely approximates to that of the reduction process described above. Therefore, the oxidation peak at +1.11 V vs. SCE could be attributed to a one-electron oxidation process. Based on the frontier orbital analysis the electron is removed from an orbital which has a major contribution from the π* C–O orbital and a minor contribution from the dyz orbital of the inner Fe atom.
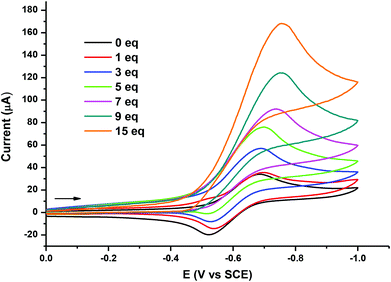
Additionally, the ability of [Fe3(CO)7(μ3-SC6H2Cl2S)2] 3 to act as an electrocatalyst for proton reduction to H2 has been evaluated. Fig. 9 provides CV responses of 3 in 0.1 M CH2Cl2/[n-Bu4N][PF6], obtained in the absence and presence of HBF4·OEt2. As can be observed, when the first 3 equivalents of HBF4·OEt2 acid were added, the reduction peak of 3 considerably increased and continued to grow in intensity with sequential addition of the acid. When 15 equivalents of the acid were added, the reduction peak reaches a maximum height, which is about seven times higher than the original reduction of 3, in the absence of the acid. At this point, when the potential scan direction is reversed, no complementary oxidation peak is observed. This behaviour is typical for a fast irreversible chemical reaction coupled to the charge-transfer step. The rapid increment in current height of the reduction peak in the presence of the acid suggests an electrocatalytic process.23 The obtained result qualitatively resembles that of related thiolate derivatives previously described by Hogarth and colleagues7 and by Pickett and colleagues8 containing tri-iron and tetra-iron assemblies, respectively.
Experimental
General
All reactions were carried out under an argon atmosphere. Solvents were dried using standard methods. Fe3(CO)12 and 1,2-dithiol HSC6H2Cl2SH are commercially available (Aldrich). [Fe2(CO)6(μ-SC6H2Cl2S)]2 and [Fe3(CO)7(μ-SCH2CH2S)2]3 were prepared according to the published procedure. The infrared spectra were recorded on a Perkin-Elmer spectrum BX FT-IR spectrophotometer using NaCl cells. 1H NMR spectra were registered on a Bruker AMX-300 instrument. Elemental analyses were performed on an LECO CHNS-932 elemental analyzer. FAB or MALDI-TOF mass spectra were carried out on a WG Autospec spectrometer or ULTRAFLEX III using 3-nitrobenzyl alcohol or DCTB as a matrix, respectively.Reaction of [Fe2(CO)6(μ-SC6H2Cl2S)] 1 with HSC6H2Cl2SH
A mixture of [Fe2(CO)6(μ-SC6H2Cl2S)] (200 mg, 0.41 mmol) and HSC6H2Cl2SH (260 mg, 1.23 mmol) in toluene (15 mL) was refluxed for 24 h. Afterwards, the dark solid formed in the reaction was filtered and recrystallized in wet acetone/n-hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at −20 °C, yielding compound [Fe2(SC6H2Cl2S)4] 2 (27 mg, 6.9%). MS (MALDI-TOF): m/z 947.5 [Fe2(SC6H2Cl2S)4]−, 473.8 [Fe(SC6H2Cl2S)2]−. Anal. Calcd for C24H8Cl8Fe2S8·4H2O·C3H6O; C, 30.07; H, 2.04; S, 23.76. Found: C, 29.08; H, 1.99; S, 24.12.
2) at −20 °C, yielding compound [Fe2(SC6H2Cl2S)4] 2 (27 mg, 6.9%). MS (MALDI-TOF): m/z 947.5 [Fe2(SC6H2Cl2S)4]−, 473.8 [Fe(SC6H2Cl2S)2]−. Anal. Calcd for C24H8Cl8Fe2S8·4H2O·C3H6O; C, 30.07; H, 2.04; S, 23.76. Found: C, 29.08; H, 1.99; S, 24.12.
Reaction of Fe3(CO)12 with HSC6H2Cl2SH
To a solution of Fe3(CO)12 (300 mg, 0.60 mmol) in toluene (15 mL), HSC6H2Cl2SH (250 mg, 1.19 mmol) was added. The resulting mixture was kept under stirring at 110 °C for 3 h. The colour of the mixture changed from deep green to brown-red. The solvent was removed under reduced pressure, and the residue was purified by column chromatography on silica gel. Elution with n-hexane gave a major first band of [Fe2(CO)6(μ-SC6H2Cl2S)] 1 (180 mg, 63%). A second band eluted with CH2Cl2 gave the brown compound [Fe3(CO)7(μ3-SC6H2Cl2S)2] 3 (30 mg, 6.4%). Elution with wet acetone yields the purple compound [Fe2(SC6H2Cl2S)4] 2 (95 mg, 17.3%).Single crystals of 3 were obtained from CH2Cl2/n-hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) at −20 °C. Spectroscopic data for compound 3: IR (hexane): νCO (cm−1) 2077 (m), 2054 (vs), 2022 (w), 2016 (s), 1999 (vw), 1977 (s). 1H NMR (CDCl3, 300 MHz, 22 °C) δ 7.10 ppm (s, 4H, C6H2). MS (FAB): m/z 781.5 [M+], 753.5-613.5 [M+ − nCO, n = 1–6]. Anal. Calcd for C19H4Cl4O7S4Fe3·2CH2Cl2; C, 26.48; H, 0.84; S, 13.45. Found: C, 26.51; H, 0.79; S, 13.11.
4) at −20 °C. Spectroscopic data for compound 3: IR (hexane): νCO (cm−1) 2077 (m), 2054 (vs), 2022 (w), 2016 (s), 1999 (vw), 1977 (s). 1H NMR (CDCl3, 300 MHz, 22 °C) δ 7.10 ppm (s, 4H, C6H2). MS (FAB): m/z 781.5 [M+], 753.5-613.5 [M+ − nCO, n = 1–6]. Anal. Calcd for C19H4Cl4O7S4Fe3·2CH2Cl2; C, 26.48; H, 0.84; S, 13.45. Found: C, 26.51; H, 0.79; S, 13.11.
Reactivity of Fe3(CO)12 with HSC6H2Cl2SH in the presence of ONMe3
To a thf (15 mL) solution of Fe3(CO)12 (150 mg, 0.3 mmol), ONMe3 (166 mg, 1.5 mmol) and HSC6H2Cl2SH (126 mg, 0.6 mmol) were added. The resulting mixture was stirred at room temperature for 20 min. Afterwards the green colour of the solution changed to brown. Then, the solvent was removed under reduced pressure and the residue was purified by column chromatography on silica gel. Elution with n-hexane gave a red band of [Fe2(CO)6(μ-SC6H2Cl2S)] 1 (11 mg, 7.7%) followed by a second brown band corresponding to compound [Fe3(CO)7(μ3-SC6H2Cl2S)2] 3 (trace amount) using CH2Cl2 as an eluent. Finally, a third purple band went down from the column using wet acetone to yield compound [Fe2(SC6H2Cl2S)4] 2 (115 mg, 40%).Mössbauer studies
Mössbauer spectra were carried out at 293 K and 77 K in triangle mode using a conventional spectrometer with the 57Co(Rh) source. The spectra were analysed by a nonlinear fit using the NORMOS program24 and the energy calibration was made using a α-Fe (6 μm) foil.Magnetic measurements
Variable temperature susceptibility measurements were carried out in the temperature range 2–300 K with an applied magnetic field of 0.1 T on ground polycrystalline samples of compound 2 (with masses of 13.79), with a Quantum Design MPMS XL-5 SQUID magnetometer. The susceptibility data were corrected for the sample holders previously measured under the same conditions and for the diamagnetic contributions of the sample using Pascal's constant tables.25Electrochemical measurements
Cyclic voltammetric (CV) experiments were recorded on a Bioanalytical Systems BASCV-50W potentiostat. CH2Cl2 (SDS, spectrograde) for electrochemical measurements was freshly distilled from calcium hydride under argon. The supporting electrolyte used was a 0.1 M solution of tetra-n-butylammonium hexafluorophosphate (Fluka), which was purified by recrystallization from ethanol and dried in a vacuum at 60 °C. A conventional three-electrode cell connected to an atmosphere of prepurified nitrogen was used. All cyclic voltammetric experiments were performed using either a platinum-disk working electrode (A = 0.020 cm2) or a glassy carbon-disk working electrode (A = 0.070 cm2) (both Bioanalytical Systems), each of which was polished on a Buehler polishing cloth with Metadi II diamond paste, rinsed thoroughly with purified water and acetone, and dried. All potentials were referenced to the saturated calomel electrode (SCE). Under our conditions, the ferrocene redox couple [FeCp2]0/+ is +0.462, and decamethylferrocene redox couple [FeCp*2]0/+ is −0.056 V vs. SCE in CH2Cl2/0.1 M n-Bu4NPF6. A coiled platinum wire was used as a counter electrode. Solutions were, typically, 10−3 M in the redox active species.X-ray structure analysis of 2 and 3
Crystals were removed from the Schlenk and covered with a layer of a viscous perfluoropolyether (Fomblin®Y). A suitable crystal was selected with the aid of a microscope, attached to a glass fiber, and immediately placed in the low temperature nitrogen stream of the diffractometer. The intensity data sets were collected at 200 K on a Bruker-Nonius KappaCCD diffractometer equipped with an Oxford Cryostream 700 unit. The structures were solved, using the WINGX package,26 by direct methods (SHELXS-97) and refined by least-squares against F2 (SHELXL-97).27 Complex 2 crystallized with four water molecules and one of acetone. Due to the poor quality of the crystal, all of them presented severe disorder and it was not possible to define a sensible chemical model, so Squeeze28 procedure was performed to remove their contribution to the structural factors. All the hydrogen atoms in both structures were positioned geometrically and refined using a riding model while all non-hydrogen atoms were anisotropically refined.Computational methods
All calculations were carried out using the DFT methodology in combination with all-electron Slater-type triple-ζ plus polarization (TZP) basis set as implemented in the ADF program.12 The OPBE functional is recommended for the calculation of Mössbauer spectroscopy and, therefore, it is employed on all calculations.18 Compounds 1 and 3 were calculated using the restricted formalism and geometrically optimized while for compound 2 the broken-symmetry unrestricted technique was employed to find the antiferromagnetic state using the X-ray structure geometry. Relativistic effects were considered except for the calculation of isomer shifts. In order to use the Noodleman fitting values the same options must be used, then relativistic effects were omitted and integration grid was lowered to 5.5.29Conclusions
The reaction between Fe3(CO)12 and HSC6H2Cl2SH has been revisited. In addition to the already reported [Fe2(CO)6(μ-SC6H2Cl2S)] 1 product, two new iron complexes, [Fe2(SC6H2Cl2S)4] 2 and [Fe3(CO)7(μ3-SC6H2Cl2S)2] 3 have been isolated. In the course of the reaction cluster 3 is converted into 2 towards the known compound 1.X-ray crystallography, Mössbauer spectroscopy and theoretical calculations have allowed unequivocally assignment of the oxidation state +III for the iron metals in compound 2. Magnetic measurements confirm a strong antiferromagnetic coupling and its magnetic moment arises from the coupling of two FeIII ions (S = 3/2) and two S = 1/2 radicals, in agreement with the DFT calculated spin electronic density for this compound.
The crystal structure of 3 shows two dithiolato ligands in an unusual triple bridging coordination mode and also represents the first example among the few related carbonyl trinuclear clusters of the group 8 metals, which contain an aromatic dithiolato ligand.
Mössbauer parameters for compounds 1, 3 and 4 have been calculated from DFT computed electronic structures and there is a very good agreement between experimental and calculated values.
Cyclic voltammetric experiments on compound 3 show a reversible reduction peak corresponding to one electron. Additionally, electrochemical results suggest that compound 3 can function, in the presence of an acid, as a catalyst for proton reduction.
Mössbauer and theoretical data do not confirm the iron oxidation states in the mixed valence cluster [Fe3(CO)7(μ3-SC6H2Cl2S)2] 3.
Acknowledgements
Financial support from Spain's Ministerio de Economía y Competitividad (MINECO) (projects CTQ2011-23157, CTQ2011-26507, CTQ2012-30728, MAT2010-20843-C02-01 and MAT2012-37109-C02-02), Generalitat Valenciana (Project Prometeo 2009/95 ISIC and ACOMP/2013/215) and Factoria de Cristalización (CONSOLIDER-INGENIO 2010) is gratefully acknowledged. We thank Dr Popescu for useful discussion on Mössbauer studies.Notes and references
- (a) N. Robertson and L. Cronin, Coord. Chem. Rev., 2002, 227, 93–127 CrossRef CAS; (b) Progress in Inorganic Chemistry, Dithiolene Chemistry: Synthesis, Properties, and Applications, ed. K. D. Karlin and E. I. Stiefel, John Wiley and Sons, New York, 2004, vol. 52 Search PubMed; (c) U. T. Muller-Westerhoff and B. Vance, in Comprehensive Coordination Chemistry, ed. G. Wilkinson, R. D. Gillard and J. A. McCleverty, Pergamon Press, Oxford, 1st edn, 1987, vol. 2 Search PubMed; (d) P. I. Clemenson, Coord. Chem. Rev., 1990, 106, 171–203 CrossRef CAS; (e) D. Belo and M. Almeida, Coord. Chem. Rev., 2010, 254, 1479–1492 CrossRef CAS PubMed; (f) S. Ezzaher, A. Gogoll, C. Bruhn and S. Ott, Chem. Commun., 2010, 5775–5777 RSC, and references herein; (g) L. Alcácer and H. Novais, in Extended Linear Chain Compounds, ed. J. S. Miller, Plenum Press, New York, 1983 Search PubMed; (h) P. Cassoux, L. Valade, H. Kobayashi, A. Kobayashi, R. A. Clark and A. E. Underhill, Coord. Chem. Rev., 1991, 110, 115–160 CrossRef CAS; (i) S. Sproules and K. Wieghardt, Coord. Chem. Rev., 2010, 254, 1358–1382 CrossRef CAS PubMed.
- L. Schwartz, P. S. Singh, L. Eriksson, R. Lomoth and S. Ott, C.R. Chim., 2008, 11, 875–889 CrossRef CAS PubMed.
- A. Winter, L. Zsolnai and G. Huttner, Z. Naturforsch., B: Anorg. Chem. Org. Chem., 1982, 37, 1430–1436 Search PubMed.
- R. D. Adams and J. H. Yamamoto, J. Cluster Sci., 1996, 7, 643–654 CrossRef CAS.
- K. H. Pannell, A. J. Mayr and D. Van Derveer, J. Am. Chem. Soc., 1983, 105, 6186–6188 CrossRef CAS.
- (a) D. Streich, M. Karnahl, Y. Astuti, C. W. Cady, L. Hammarrstrom, R. Lomoth and S. Ott, Eur. J. Inorg. Chem., 2011, 1106–1111 CrossRef CAS, and references therein; (b) R. J. Wright, C. Lim and T. D. Tilley, Chem. – Eur. J., 2009, 15, 8518–8525 CrossRef CAS PubMed; (c) G. Durgaprasad, R. Bolligarla and S. K. Das, J. Organomet. Chem., 2012, 706–707, 37–45 CrossRef CAS PubMed; (d) U. P. Apfel, D. Troegel, Y. Halpin, S. Tschierlei, U. Uhlemann, H. Georls, M. Schmitt, J. Popp, P. Dunne, M. Venkatesan, M. Coey, M. Rudolph, J. G. Vos, R. Tacke and W. Weigand, Inorg. Chem., 2010, 49, 10117–10132 CrossRef CAS PubMed; (e) C. Tard and C. J. Pickett, Chem. Rev., 2009, 109, 2245–2274 CrossRef CAS PubMed.
- S. Ghosh, G. Hogarth, K. B. Holt, S. E. Kabir, A. Rahaman and D. G. Unwin, Chem. Commun., 2011, 11222–11224 RSC.
- C. Tard, X. Liu, D. L. Hughes and C. J. Pickett, Chem. Commun., 2005, 133–135 RSC.
- P. Amo-Ochoa, E. Delgado, C. J. Gómez-García, D. Hernández, E. Hernández, A. Martin and F. Zamora, Inorg. Chem., 2013, 52, 5943–5950 CrossRef CAS PubMed.
- A. K. Patra, E. Bill, T. Weyhermüller, K. Stobie, Z. Bell, M. D. Ward, J. A. McCleverty and K. Wieghardt, Inorg. Chem., 2006, 45, 6541–6548 CrossRef CAS PubMed.
- R. Yu, K. Arumugam, A. Manepalli, Y. Tran, R. Schmehl, H. Jacobsen and J. P. Donahue, Inorg. Chem., 2007, 46, 5131–5133 CrossRef CAS PubMed.
- (a) ADF2010.02; SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands Search PubMed; (b) G. te Velde, F. M. Bickelhaupt, E. J. Baerends, C. Fonseca Guerra, S. J. A. Van Gisbergen, J. G. Snijders and T. Ziegler, J. Comput. Chem., 2001, 22, 931–967 CrossRef CAS.
- J. J. Borrás-Almenar, J. M. Clemente-Juan, E. Coronado and B. S. Tsukerblat, Inorg. Chem., 1999, 38, 6081–6088 CrossRef PubMed.
- J. J. Borrás-Almenar, J. M. Clemente-Juan, E. Coronado and B. S. Tsukerblat, J. Comput. Chem., 2001, 22, 985–991 CrossRef.
- M. Hu, C. Ma, H. Wen, H. Cui and C. Chen, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2014, 70, m124 CAS.
- N. Begum, M. I. Hyder, S. E. Kabir, G. M. G. Hossain, E. Nordlander, D. Rokhsana and E. Rosenberg, Inorg. Chem., 2005, 44, 9887–9894 CrossRef CAS PubMed.
- R. D. Adams, L. Chen and J. H. Yamamoto, Inorg. Chim. Acta, 1995, 229, 47–54 CrossRef CAS.
- (a) N. C. Handy and A. J. Cohen, Mol. Phys., 2001, 99, 403–412 CrossRef CAS; (b) J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 785–789 CrossRef; (c) J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1997, 78, 1396 CrossRef CAS.
- C.-H. Hsieh, O. F. Erdem, S. D. Harmann, M. L. Singleton, E. Reijerse, W. Lubitz, C. V. Popescu, J. H. Reibenspies, S. M. Brothers, M. B. Hall and M. Y. Darensbourg, J. Am. Chem. Soc., 2012, 134, 13089–13102 CrossRef CAS PubMed.
- S. A. Stoian, Ch.-H. Hsieh, M. L. Singleton, A. F. Casuras, M. Y. Darensbourg, K. McNeely, K. Sweely and C. V. Popescu, J. Biol. Inorg. Chem., 2013, 18, 609–622 CrossRef CAS PubMed.
- Electrochemical Methods, ed. A. J. Bard and L. R. Faulkner, John Wiley & Sons, New York, 1980 Search PubMed.
- Inorganic Electrochemistry. Theory, Practice and Application, ed. P. Zanello, C. Nervi and F. Fabrizi de Biani, Royal Society of Chemistry, Cambridge, 2nd edn, 2012 Search PubMed.
- (a) I. Bhugun, D. Lexa and J.-M. Saveant, J. Am. Chem. Soc., 1996, 118, 3982–3983 CrossRef CAS; (b) S. J. Borg, T. Behrsing, S. P. Best, M. Razavet, X. Liu and C. J. Pickett, J. Am. Chem. Soc., 2004, 126, 16988–16999 CrossRef CAS PubMed.
- R. A. Brand, Nucl. Instrum. Methods Phys. Res., Sect. B, 1987, 28, 398–416 CrossRef.
- G. A. Bain and J. F. Berry, J. Chem. Educ., 2008, 85, 532–536 CrossRef CAS.
- L. J. Farrugia, J. Appl. Crystallogr., 1999, 32, 837–838 CrossRef CAS.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
- P. Van der Sluis and A. L. Spek, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 1990, 46, 194–201 CrossRef.
- G. M. Sandala, K. H. Hopmann, A. Ghosh and L. Noodleman, J. Chem. Theor. Comput., 2011, 7, 3232–3247 CrossRef CAS PubMed.
Footnotes |
| † Dedicated to Professor David Cole-Hamilton on the occasion of his retirement and for his outstanding contribution to transition metal catalysis. |
| ‡ Electronic supplementary information (ESI) available: Additional experimental and theoretical data. CCDC 1002771 (2) and 1002772 (3). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4dt01462f |
| This journal is © The Royal Society of Chemistry 2014 |