Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Homo- and heteroleptic alkoxycarbene f-element complexes and their reactivity towards acidic N–H and C–H bonds†
Polly L.
Arnold
*a,
Thomas
Cadenbach
a,
Isobel H.
Marr
a,
Andrew A.
Fyfe
a,
Nicola L.
Bell
a,
Ronan
Bellabarba
b,
Robert P.
Tooze
b and
Jason B.
Love
*a
aEaStCHEM School of Chemistry, University of Edinburgh, The King's Buildings, Edinburgh, EH9 3JJ, UK. E-mail: jason.love@ed.ac.uk; polly.arnold@ed.ac.uk
bSasol Technology UK, Purdie Building, North Haugh, St Andrews, KY16 9SR, UK
First published on 11th June 2014
Abstract
The reactivity of a series of organometallic rare earth and actinide complexes with hemilabile NHC-ligands towards substrates with acidic C–H and N–H bonds is described. The synthesis, characterisation and X-ray structures of the new heteroleptic mono- and bis(NHC) cyclopentadienyl complexes LnCp2(L) 1 (Ln = Sc, Y, Ce; L = alkoxy-tethered carbene [OCMe2CH2(1-C{NCHCHNiPr})]), LnCp(L)2 (Ln = Y) 2, and the homoleptic tetrakis(NHC) complex Th(L)44 are described. The reactivity of these complexes, and of the homoleptic complexes Ln(L)3 (Ln = Sc 3, Ce), with E–H substrates is described, where EH = pyrrole C4H4NH, indole C8H6NH, diphenylacetone Ph2CC(O)Me, terminal alkynes RC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH (R = Me3Si, Ph), and cyclopentadiene C5H6. Complex 1-Y heterolytically cleaves and adds pyrrole and indole N–H across the metal carbene bond, whereas 1-Ce does not, although 3 and 4 form H-bonded adducts. Complexes 1-Y and 1-Sc form adducts with CpH without cleaving the acidic C–H bond, 1-Ce cleaves the Cp–H bond, but 2 reacts to form the very rare H+–[C5H5]−–H+ motif. Complex 1-Ce cleaves alkyne C–H bonds but the products rearrange upon formation, while complex 1-Y cleaves the C–H bond in diphenylacetone forming a product which rearranges to the Y–O bonded enolate product.
CH (R = Me3Si, Ph), and cyclopentadiene C5H6. Complex 1-Y heterolytically cleaves and adds pyrrole and indole N–H across the metal carbene bond, whereas 1-Ce does not, although 3 and 4 form H-bonded adducts. Complexes 1-Y and 1-Sc form adducts with CpH without cleaving the acidic C–H bond, 1-Ce cleaves the Cp–H bond, but 2 reacts to form the very rare H+–[C5H5]−–H+ motif. Complex 1-Ce cleaves alkyne C–H bonds but the products rearrange upon formation, while complex 1-Y cleaves the C–H bond in diphenylacetone forming a product which rearranges to the Y–O bonded enolate product.
Introduction
N-heterocyclic carbenes (NHCs) have found widespread application in many areas of molecular chemistry, as nucleophilic reagents in organic transformations1–3 and as strongly Lewis basic σ-donor ligands in both transition metal4–6 and rare-earth metal chemistry.7–11 As organocatalysts, NHCs are mainly used either due to their strong nucleophilicity, for example as initiators by the umpolung of electrophilic carbonyl groups into nucleophilic acyl anion equivalents for benzoin condensation12,13 and the Stetter reaction,14,15 or Brønsted basicity in stoichiometric and catalytic transesterification16–18 and acylation19–21 reactions. In late transition metal chemistry, NHCs are extensively exploited as ligands for homogeneous catalysts in alkene metathesis and C–C coupling reactions.4–6,22In contrast, the use of neutral NHCs as donor-ligands for the rare earth elements is less common due to a mismatch in the bonding to the hard Lewis acid, resulting in inopportune lability of the NHC in these complexes.8,10,11 Metallocenes of the rare earth metals are known to activate C–H bonds in both saturated and unsaturated organic substrates through σ-bond metathesis mechanisms rather than the conventional two-electron oxidative addition–reductive elimination pathway seen for late transition metals.23–25 Furthermore, organometallic complexes of f-block metals such as alkyls, hydrides, and amido compounds are excellent catalysts for the hydroamination, phosphination, alkoxylation, and silylation of alkenes.26–31
Recently, we and others became interested to combine the advantages of NHC ligands with the beneficial properties of rare earth metals.8,10,11 In order to make use of the relative lability of the NHC ligand in f-block compounds, we exploited anionic O- or N-moieties to tether these ligands to the Lewis acidic metal. Accordingly, these tethered NHC ligands can function as reactive ligands, and the combination of the Lewis acidic metal cation and nucleophilic carbene ligand has led us to compare the reactivity of these complexes to frustrated Lewis pairs.32,33 For example, we have shown that polar substrates can be cleaved heterolytically, in a reversible manner, across the metal–NHC bond. Subsequent heating of these addition products led to an elimination of the functionalised substrate from the lanthanide complex, reforming the metal–carbene bond. This reactivity allowed us to form new C–C, C–E, and N–E (E = Si, P, B or Sn) bonds at redox innocent metals.34,35
In order to gain a deeper insight into these reactions and to broaden the substrate scope we were interested in the reactivity of electropositive metal NHC complexes towards substrates with acidic C–H and N–H bonds in order to avoid the incorporation of halide or pseudo-halide anions. In this context, N-heterocycles such as those based on pyrrole and indole are of particular interest due to their widespread application in natural products, pharmaceutical agents and materials science.36 Thus, their functionalization has been subject of numerous studies. Additionally, due to their flexible binding mode, pyrrole and indole anions have found use as important ligands in transition metal and rare-earth metal chemistry.36–43 For example, it was shown that rare-earth metal complexes of functionalized indolyl ligands are catalysts for olefin polymerization.44
Herein, we describe the syntheses of heteroleptic mono- and bis(NHC) cyclopentadienyl, as well as homoleptic tris- and tetrakis(NHC) f-block metal complexes and their reactivity towards N–H and C–H acidic substrates.
Results and discussion
Syntheses of mono-, bis-, tris- and tetrakis(carbene) metal complexes
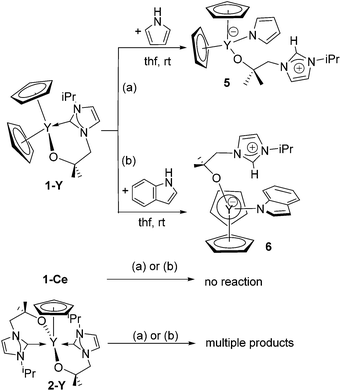
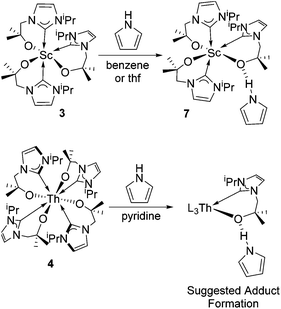
We identified tris(cyclopentadienyl) complexes as potential synthons for mono- and bis(carbene) cyclopentadienyl rare-earth complexes as the cyclopentadienyl salt should be readily eliminated. Reaction between YCp3 and one equivalent of KL, K[OCMe2CH2(1-C{NCHCHNiPr})], in THF at 60 °C affords colourless crystals of YCp2(L) 1-Y in good yield (78%) after work-up (Scheme 1). Using a similar approach, the scandium and cerium compounds ScCp2(L) 1-Sc and CeCp2(L) 1-Ce were isolated. Compounds 1-Sc, 1-Y, 1-Ce were characterized fully, in particular by NMR spectroscopy and single crystal X-ray diffraction (see below). Furthermore, the reaction of YCp3 and two equivalents of KL allows the isolation of YCp(L)22-Y in good yield (71%); this complex can also be prepared by the reaction of 1-Y with KL. Single crystals of 2-Y suitable for X-ray crystallography were grown from a concentrated thf solution. The mono(carbene)bis(cyclopentadienyl) compounds can also be made by salt metathesis from the corresponding bis(cyclopentadienyl) chloride complexes LnCp2(Cl) and KL in THF at 60 °C, but lower yields are isolated from this route; the ready availability of LnCp3 starting materials renders the former synthetic route preferable.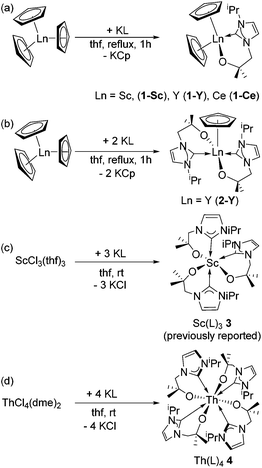 | ||
| Scheme 1 Syntheses of (a) 1-Sc, 1-Y, 1-Ce and (b) 2-Y by KCp elimination, and of the complexes 3 (c, previously reported)32 and 4 (d) by salt metathesis routes. | ||
The NMR spectra of all compounds 1 and 2 indicate that the structures are rigid in solution at room temperature with the carbene ligands bound to the metal centres. The 13C NMR spectrum of 1-Y in C6D6 shows a doublet at 192.1 ppm with a coupling constant of 47.6 Hz; complexes containing yttrium-bound carbenes have chemical shifts in the range of 186–197 ppm. The 1H NMR spectrum of 1-Y contains, as expected, a single resonance for both cyclopentadienyl ligands at 6.21 ppm as well as a set of resonances for the NHC ligand. The most characteristic resonances of the alkoxy tethered carbene are defined by the two backbone protons of the imidazolium ring at 6.13 (JHH = 1.70) and 6.09 (JHH = 1.61 Hz) as well as a septet for the single isopropyl H at 4.18 ppm (JHH = 6.76 Hz).
The 1H NMR spectrum of 1-Ce shows a set of paramagnetically contact-shifted ligand resonances between 20 and −5 ppm. The 1H NMR spectrum of 2-Y in C6D6 shows a single set of signals for both NHC ligands and a corresponding singlet for the cyclopentadienyl ligand at 6.53 ppm. As indicated by the higher frequency of the Cp resonance, the resonances due to the NHC groups are also shifted to higher frequency at 6.36 (doublet for backbone NHC protons, JHH = 1.50 Hz), 6.20 (doublet for backbone NHC protons, JHH = 1.38 Hz) and 5.73 ppm (septet for single isopropyl proton, JHH = 6.77 Hz). The 13C{1H} NMR spectrum of 2-Y contains a doublet for the yttrium-bound carbene at 194.8 ppm with a coupling constant of 1JYC = 37.3 Hz.
We recently reported the synthesis of Sc(L)33 (Scheme 1),32 and now show that Th(L)44, can be isolated from a similar room-temperature reaction between ThCl4(dme)2 and four equivalents of KL in THF for one hour. The 1H NMR spectrum of 4 shows broad resonances for all of the ligand protons at room temperature, indicative of a dynamic process occurring in solution. Two sharper resonances at 6.53 and 6.38 ppm are identified as the imidazole CH groups but the isopropyl CH is a very broad singlet at 6.02 ppm (Fig. 1). At 50 °C, the 1H NMR spectrum shows a significant sharpening of the ligand resonances with the septet of the isopropyl methine proton now clearly visible at 5.88 ppm along with a singlet (2H) at 3.81 ppm. On cooling, the 1H NMR spectra display coalescence, with, at −60 °C, two ligand environments present in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with equal-intensity resonances at 6.85, 6.71, 6.62 and 6.49 ppm for the imidazole CH protons, and two isopropyl septet resonances clearly visible at 6.09 and 5.71 ppm. The methylene protons from the alkoxide tether now appear as four doublets integrating for 1H each; although one of these latter resonances is obscured by the thf co-solvent its presence can be inferred, while the others appear at 4.79, 3.19 and 3.03 ppm. The two similar ligand environments, each with diastereotopic CH2 groups are most sensibly assignable to a static, eight-coordinate structure. In this structure, the carbenes are bound to the Th centre with two magnetically non-equivalent, bidentate ligand environments (arising from slight twisting of each ligand), and diastereotopic CH2 groups in each that give rise to four non-equivalent CH resonances. This assignment of the low-temperature limiting solution structure of 4 is supported by its X-ray crystal structure (see below).
1 ratio with equal-intensity resonances at 6.85, 6.71, 6.62 and 6.49 ppm for the imidazole CH protons, and two isopropyl septet resonances clearly visible at 6.09 and 5.71 ppm. The methylene protons from the alkoxide tether now appear as four doublets integrating for 1H each; although one of these latter resonances is obscured by the thf co-solvent its presence can be inferred, while the others appear at 4.79, 3.19 and 3.03 ppm. The two similar ligand environments, each with diastereotopic CH2 groups are most sensibly assignable to a static, eight-coordinate structure. In this structure, the carbenes are bound to the Th centre with two magnetically non-equivalent, bidentate ligand environments (arising from slight twisting of each ligand), and diastereotopic CH2 groups in each that give rise to four non-equivalent CH resonances. This assignment of the low-temperature limiting solution structure of 4 is supported by its X-ray crystal structure (see below).
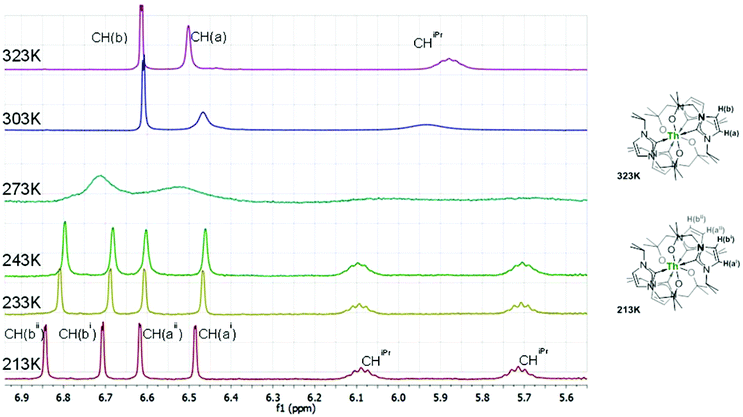 | ||
| Fig. 1 Variable temperature NMR spectra for Th(L)44 in C6D6–THF (range 5.5–7.3 ppm) showing the non-equivalence of the ligands at low temperature (diastereotopic CH2 groups are not displayed). | ||
X-ray single crystal structures of mono-, bis-, tris and tetrakis(carbene) metal complexes
Compounds 1-Sc and 1-Y share the same structural motif in the solid state (Fig. 2 and Table 1), displaying monomeric structures in which the metal centres are coordinated in a pseudo-tetrahedral environment by two cyclopentadienyl ligands and the alkoxide and carbene donors of the bidentate NHC ligand. Crystallization of 1-Ce from a concentrated thf solution affords crystals of 1-Ce·THF, in which a molecule of THF is bound to the f-element centre and generates a distorted pseudo-trigonal bipyramidal coordination geometry with the bidentate NHC ligand and one Cp defining the equatorial plane. In all of these complexes, the cyclopentadienyl ligands are η5 coordinated with M–Cp bond distances in the expected range. Within the series of bis(cyclopentadienyl) complexes 1-Sc, 1-Y and 1-Ce, the M–CpCt (CpCt = Cp ring centroid), M–O and M–Ccarbene bond distances increase in the order Sc < Y < Ce, due to the increasing ionic radii and electropositive character of the corresponding metal centre. The M–Ccarbene bond distances in 1-Sc and 1-Y of 2.337(2) and 2.489(5) Å, respectively, are noticeably shorter than those found in the homoleptic complexes Sc(L)332 (mean Sc–Ccarbene 2.422 Å) and Y(L)345 (mean Y–Ccarbene 2.588(12) Å) and are generally short, but still comparable with other Sc–NHC and Y–NHC complexes. For example, these distances in Ln(CH2SiMe3)2(Ind-CH2CH2{1-C(NCHCHNMes)}) (Ind = indenyl) are 2.350(3) Å (Ln = Sc) and 2.502(1) Å (Ln = Y).46 However, it should be noted that, to our knowledge, the Y–C bond of 2.489(5) Å in 1-Y is the shortest Y–NHC distance yet reported. Similarly, the M–O bonds in 1-Sc and 1-Y of 1.952(2) and 2.091(4) Å, respectively, are slightly shorter compared to those found in the homoleptic complexes Sc(L)3 (Sc–O 1.989(2)–2.046(2) Å) and Y(L)345 (Y–O 2.115(3)–2.179(7) Å).32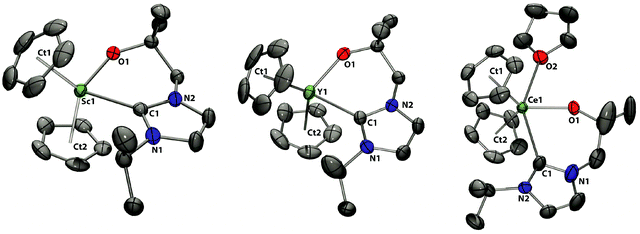 | ||
| Fig. 2 Solid state structures of 1-Sc, 1-Y, 1-Ce. For clarity, H atoms are omitted (displacement ellipsoids drawn at 50% probability). | ||
| 1-Sc | 1-Y | 1-Ce | 2-Y | |
|---|---|---|---|---|
| M–Ccarbene | 2.337(2) | 2.489(5) | 2.735(4) | 2.568(3) |
| 2.597(3) | ||||
| M–Cpct | 2.247, 2.261 | 2.387, 2.411 | 2.590, 2.597 | 2.442 |
| M–O | 1.952(2) | 2.091(4) | 2.172(2) | 2.1066(18) |
| 2.1201(18) | ||||
| Ccarbene–N(1) | 1.358(3) | 1.349(6) | 1.364(5) | 1.359(3) |
| Ccarbene–N(2) | 1.359(3) | 1.356(6) | 1.350(5) | 1.364(3) |
| 1-Sc | 1-Y | 1-Ce | 2-Y | |
| Cpct–M–Cpct | 126.22 | 130.15 | 122.76 | — |
| N–Ccarbene–N | 103.5(2) | 102.9(2) | ||
| 103.4(2) | ||||
| O–M–Ccarbene | 83.78(7) | 79.90(15) | 72.61(11) | 75.68(8) |
| 90.45(8) | ||||
| CpCt1–M–O | 113.50 | 110.37 | 117.11–118.63 | |
| CpCt1–M–Ccarbene | 109.71 | 111.88 | 101.06 | 107.17–108.09 |
| CpCt2–M–Ccarbene | 99.56. | 100.06 | 96.87 | — |
The Ce–Ccarbene bond length in 1-Ce of 2.735(4) Å is slightly longer than in Ce(N{SiMe3}2)2(C{N(tBu)CHCHN}–CH2CH2NtBu)47 and similar to those in the CeIV complex Ce(L)448 (mean Ce–Ccarbene 2.674(7) Å). The slightly elongated Ce–Ccarbene distance can be explained by the coordination of a THF molecule and the resulting steric crowding in five coordinate 1-Ce compared to the four coordinate amidocarbene complex.
Compound 2-Y crystallized with two independent molecules in the asymmetric unit; since both have very similar geometrical parameters, only one is discussed here (Fig. 3). The yttrium centre in compound 2-Y is in a distorted trigonal bipyramidal coordination environment with both alkoxides and the cyclopentadienyl group in the equatorial plane and the carbene donors axial. The Y–Ccarbene bond lengths are 2.568(3) to 2.597(3) Å with a mean distance of 2.582 Å. Comparison of 2-Y with 1-Y shows that the Y–Ccarbene as well as the Y–O (mean 2112(4) Å) bond distances in 2-Y are longer and now very similar to those found in Y(L)3. The shorter M–Ccarbene and M–O distances in 1-Sc and 1-Y can be attributed to the more electrophilic character of the metal centres due to the weaker electron donating ability of the Cp rings in 1-Sc and 1-Y compared to the alkoxide groups in the homoleptic compounds Sc(L)3 and Y(L)3. Accordingly, replacement of one cyclopentadienyl ligand in 1-Y by one bidentate NHC ligand leads to the elongation of the Y–O and Y–Ccarbene distances in 2-Y (Fig. 2 and 3).
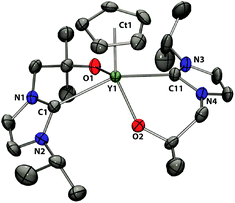 | ||
| Fig. 3 Solid state structure of 2-Y. For clarity, H atoms are omitted (displacement ellipsoids drawn at 50% probability). | ||
The solid-state structure of Th(L)4 (Fig. 4) shows four bidentate ligands coordinated in a mutually head-to-tail fashion, forming a square-antiprismatic Th coordination sphere. This contrasts with the solid state structures of Ce(L)4 and U(L)4 which exhibit 6 and 7-coordinate geometries respectively reflecting the large ionic radius of Th(IV) at 119 pm (cf. Ce(IV) 111 pm and U(IV) 114 pm).48,49 The slight twist in ligand backbone affords two slightly different ligand geometries, which are reflected by the torsion angles of 25.1° for O3–Th–C3–N3 and −20.4° for O2–Th–C2–N2. However, the difference between the two ligand environments is not sufficient to be manifested in different bond distances between the two forms, within standard uncertainties. The Th–C bonds are in the range 2.852(6) to 2.884(5) Å, all significantly longer than the longest direct Th–C bond yet reported of 2.77 Å, and reflect the weakness of the carbene–actinide interaction. The Th–O bonds are all slightly shorter than the average thorium alkoxide/aryloxide bond (2.422 Å) but within the expected range (2.1–2.7 Å).
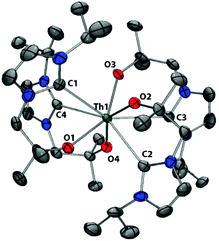 | ||
| Fig. 4 Solid state structure of 4. For clarity, H atoms are omitted (displacement ellipsoids drawn at 50% probability). | ||
Reactivity
The reactivity of the series of homoleptic and heteroleptic complexes described above was studied with range of substrates that contain acidic C–H and N–H bonds.N–H acidic substrates – pyrrole and indole
The reactions between heteroleptic and homoleptic carbene complexes 1-M, 2-Y, 3 and 4 and pyrrole or indole were carried out (Scheme 2). Reaction of a solution of 1-Y with 1 equivalent of pyrrole or indole in benzene or thf at room temperature immediately results in the formation of a colourless precipitate. In each case, the complexes formulated as Cp2Y[H{C(iPrNCH2CH2N)}CH2CMe2O]-(L′) (L′ = C4H4N for 5 and C8H6N for 6) are isolated in good yield. This reaction can formally be seen as an acid–base reaction in which the NHC ligand acts as a base by deprotonating the acidic N–H of the pyrrole (pKa = 23.0 in dmso)50 or indole (pKa = 21.0 in dmso),50 resulting in a pendant imidazolium group. The resulting nucleophilic pyrrolide and indolide anions are then trapped at the Lewis acidic yttrium centre forming the corresponding compounds 5 and 6. This reaction can also be described as the heterolytic addition of the polar N–H substrates pyrrole and indole across the Y–Ccarbene bond, forming a strong Y–N bond and an imidazolium cation. The resulting zwitterionic compounds are stable even at elevated temperatures. No reaction between N-methylindole and 1-Y was observed, which rules out the possibility of a C–H bond cleavage reaction at the C3 position of indole followed by a subsequent rearrangement to yield 6.Complexes 5 and 6 are insoluble in non-polar solvents such as hexane, benzene or toluene and slightly soluble in polar solvents such as thf, dioxane and pyridine. However, it should be noted that compounds 5 and 6 decompose slowly in pyridine solutions. Moreover, both compounds are extremely reactive towards water, liberating free pyrrole and indole upon reaction. The 1H NMR spectrum of 5 in d8-thf contains a single resonance for both cyclopentadienyl ligands at 5.89 ppm. The characteristic resonance for the imidazolium proton is present at 6.32 ppm whereas the other resonances of the alkoxide arm are slightly shifted when compared to the starting material 1-Y. The pyrrolide group shows resonances at 6.86 and 5.89 ppm. Similarly, the 1H NMR spectrum of 6 in d8-thf shows a single resonance for both cyclopentadienyl ligands at 5.92 ppm. The signal for the imidazolium proton is significantly shifted to higher frequency at 7.37 ppm. Furthermore, the characteristic septet for the isopropyl proton of the alkoxy-tethered carbene at 3.24 ppm is shifted to lower frequency when compared to 1-Y (δ = 4.54 ppm) and 5 (δ = 4.46 ppm), respectively.
Surprisingly, when a thf solution of the cerium analogue 1-Ce is mixed with pyrrole or indole, no reaction occurs. Moreover, reaction of pyrrole and indole with the bis(carbene) 2-Y leads to unidentifiable multiple products in both cases as evidenced by 1H NMR spectroscopy. All attempts to drive the reaction towards one specific product by variation of reaction conditions such as time, temperature, rate of addition, and reagent concentration leads to same result, i.e. formation of multiple reaction products.
The reaction of 3 with one equivalent of pyrrole results in the formation of the pyrrole adduct Sc(L)3·HNC4H47 (Scheme 3), in which the pyrrole N–H hydrogen bonds with one alkoxide tether. The 1H NMR of 7 in C6D6 shows one set of broad resonances for all three carbene ligands. Additionally, the spectrum contains a characteristic resonance for the pyrrole N–H at 11.61 ppm, which is shifted to higher frequency by 4.6 ppm from the chemical shift of free pyrrole, indicative of a strong hydrogen-bonding interaction. The ortho-C–H resonances are also shifted by 0.4 ppm. Furthermore, the 13C NMR spectrum contains a single peak at δ = 194.7 ppm indicating that the scandium–carbene bonds are intact. It should be noted that removal of the solvent and drying the resulting crude material under vacuum leads to the loss of pyrrole and to the reformation of 3.
Treatment of a pyridine solution of the homoleptic Th complex 4 with one equivalent of pyrrole results in a broadening of the ligand resonances for 4 although no distinct new species was observed. The 1H NMR in d5-pyridine contains a characteristic resonance for the pyrrole N–H at 10.18 ppm, which is shifted to higher frequency from free pyrrole, suggesting that a similar hydrogen bonding interaction to that in 7 is occurring. Also, the ortho-C–H resonances are shifted by 0.24 ppm and the backbone C–H resonances are shifted by 0.14 ppm. Treatment of the reaction mixture with a further excess of pyrrole results in the sharpening of the ligand resonances and disappearance of the resonances of 4. At least two ligand environments are evident in the 1H NMR spectrum with two septets corresponding to the isopropyl CHs at 4.50 ppm and 4.42 ppm. Based on the integration of these multiplets it appears there are at least two different species in solution. Removal of the solvent and pyrrole under reduced pressure results in the reformation of 4 suggesting that while an interaction with pyrrole occurs, it is not stable towards loss of pyrrole and supports a structure in which only hydrogen-bonding interactions between the Th-alkoxide and the pyrrole N–H occur.
Single crystals of 5, 6, and 7 were grown and the solid state structures determined by X-ray diffraction (Fig. 5 and 6). Each metal centre in 5 and 6 is four-coordinate with two η5-cyclopentadienyl ligands, the protonated alkoxy-tethered NHC ligand and a κ1-N–C4H4N (5) or κ1-N–C8H6N (6) ligand. The coordination environment in each complex displays a slightly distorted tetrahedral geometry with average tetrahedral angles of 108.6° for 5 and 108.8° for 6. The Y–N bond distance for the pyrrolide ligand in 5 is 2.3611(19) Å whereas the Y–N bond distance for the indolide ligand in 6 is 2.376(3) Å. These values are within the expected range for Y–N bond lengths such as 2.337(2) Å in [Y(η5:η1-C5Me4SiMe2NCMe3)(κ1-N–C4H4N)(dme)].51 The Y–CpCt distances in 5 and 6 remain unchanged when compared to the starting compound 1-Y. As indicated by the corresponding 1H NMR spectra, the carbene ligands in 5 and 6 are protonated with the resulting imidazolium protons directed towards the electron-rich, anionic nitrogen donor, so forming internal hydrogen-bonding interactions (5: C3⋯N1 = 3.4330(1) Å; 6: C15⋯N3 = 3.4957(4) Å).52
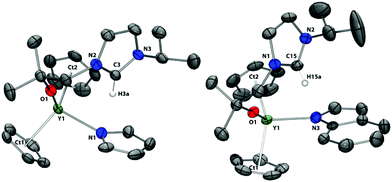 | ||
| Fig. 5 Solid state structures of 5 and 6. For clarity, H atoms, except the imidazolium proton, are omitted (displacement ellipsoids drawn at 50% probability). | ||
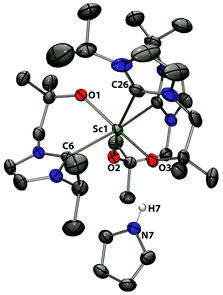 | ||
| Fig. 6 Solid state structure of 7. For clarity, H atoms, except the pyrrole proton, are omitted (displacement ellipsoids drawn at 50% probability). | ||
The Sc centre in 7 is coordinated by three bidentate, alkoxy-tethered carbene ligands in a pseudo-octahedral environment, adopting a mer geometry. The mean Sc–Ccarbene bond distance is 2.423 Å with a range of 2.4094(14) Å to 2.4509(15) Å and thus almost identical to those observed in the parent compound 3 and comparable to those found in other Sc–NHC complexes, e.g. in Sc(CH2SiMe3)(LD)2 (where LD = ({C(NDippCH2CH2N)}–CH2CMe2O)).35 Similarly, the Sc1–C26 bond of 2.4509(15) Å between the metal and the NHC trans to the alkoxide is notably longer than the other two Sc–Ccarbene bond distances of 2.4099(14) and 2.4094(14) Å. The most striking feature in the solid state structure of 7 is the pyrrole group which hydrogen bonds through its N–H proton to the alkoxide tether O3. The N7⋯O3 separation is 2.7653(16) Å and thus comparable with other complexes containing pyrrole hydrogen-bond interactions.
Acidic C–H bonds – alkynes
In order to test the reactivity of this series of homo- and heteroleptic metal carbene complexes towards acidic C–H bonds, reactions were carried out with various alkynes RC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH (with R = Me3Si, Ph, tBu). The pKa values of these C–H bonds in these alkynes are in the range 25–29, with a precise value of 28.8 reported for R = Ph in dmso.53 None of the homoleptic alkoxy-carbene complexes 3, 4, Ce(L)348 or the potassium salt KL showed any evidence of reaction; nor did the heteroleptic carbene complexes 1-Sc, 1-Y and 2-Y.
CH (with R = Me3Si, Ph, tBu). The pKa values of these C–H bonds in these alkynes are in the range 25–29, with a precise value of 28.8 reported for R = Ph in dmso.53 None of the homoleptic alkoxy-carbene complexes 3, 4, Ce(L)348 or the potassium salt KL showed any evidence of reaction; nor did the heteroleptic carbene complexes 1-Sc, 1-Y and 2-Y.
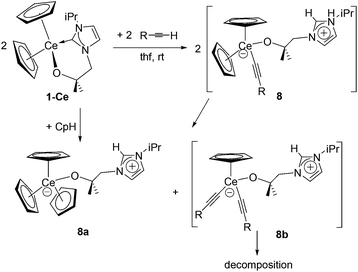
However, the reaction of a thf or benzene solution of 1-Ce with one equivalent of a terminal alkyne RC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CH (R = Me3Si, Ph) at room temperature resulted in a colour change from pale yellow to dark red, followed by a rapid formation of large colourless crystals which were characterised as CeCp3(LH) 8a (Scheme 4). This product presumably arises from ligand redistribution within the initially formed product, tentatively assigned as CeCp2(HL)(C
CH (R = Me3Si, Ph) at room temperature resulted in a colour change from pale yellow to dark red, followed by a rapid formation of large colourless crystals which were characterised as CeCp3(LH) 8a (Scheme 4). This product presumably arises from ligand redistribution within the initially formed product, tentatively assigned as CeCp2(HL)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR) 8, which rearranges to the sterically protected and highly crystalline 8a and the alkynide CeCp(HL)(C
CR) 8, which rearranges to the sterically protected and highly crystalline 8a and the alkynide CeCp(HL)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR)28b, which presumably oligomerises or polymerises to an insoluble material due to the high degree of steric unsaturation. The solubility of 8a is poor in organic solvents, and although a set of broadened, paramagnetically contact-shifted resonances can be assigned in its 1H NMR spectrum, no definitive identification of the imidazolium CH resonance is possible. A few different reaction conditions were carried out in order to attempt to isolate the by-product 8b or to characterise it in situ by NMR or FTIR spectroscopy, but all were unsuccessful. To our knowledge, no kinetically inert σ-bonded cerium alkynide complexes have yet been reported. Single crystals of 8a were analysed by X-ray diffraction, further confirming its identity (see below, Fig. 7).
CR)28b, which presumably oligomerises or polymerises to an insoluble material due to the high degree of steric unsaturation. The solubility of 8a is poor in organic solvents, and although a set of broadened, paramagnetically contact-shifted resonances can be assigned in its 1H NMR spectrum, no definitive identification of the imidazolium CH resonance is possible. A few different reaction conditions were carried out in order to attempt to isolate the by-product 8b or to characterise it in situ by NMR or FTIR spectroscopy, but all were unsuccessful. To our knowledge, no kinetically inert σ-bonded cerium alkynide complexes have yet been reported. Single crystals of 8a were analysed by X-ray diffraction, further confirming its identity (see below, Fig. 7).
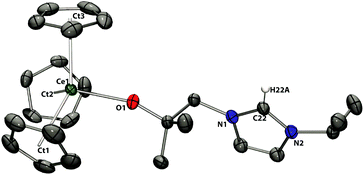 | ||
| Fig. 7 Solid state structure of 8a. For clarity, H atoms, except the imidazolium proton, are omitted (displacement ellipsoids drawn at 50% probability). | ||
Acidic C–H bonds – cyclopentadiene
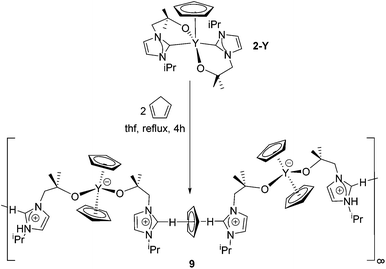
The reaction of 1-Ce with one equivalent of freshly distilled cyclopentadiene results in the clean formation of 8a in good yield (Scheme 4). This demonstrates the reactive, non-innocent character of the carbene ligand which acts as a base in order to activate the C–H acidic substrate cyclopentadiene (pKa CpH = 18.0).54 On the other hand, monitoring a reaction between either 1-Y or 1-Sc and freshly distilled cyclopentadiene in benzene or thf solution by 1H NMR spectroscopy shows that these congeners do not react to give the yttrium or scandium analogues, i.e. Cp3Y(HL) or Cp3Sc(HL), respectively. This might be a consequence of the smaller size of these two cations compared with Ce. However, it is of interest to note that after heating the mixture of 1-Y and cyclopentadiene for five days at 60 °C, both reactants were completely unchanged, i.e. the anticipated dimerization of cyclopentadiene to dicyclopentadiene had not occurred. This suggests that perhaps there is indeed an addition reaction taking place with cyclopentadiene but it is in a rapid equilibrium in solution, resulting in no net product formation but no substrate ‘decomposition’.The reaction between 2-Y and two equivalents of freshly distilled cyclopentadiene at 60 °C for one hour results in the deprotonation of both cyclopentadiene molecules and in the formation of the polymer [YCp2(LH)2(Cp)]∞9 (Scheme 5). Analogous in part to the reaction of 1-Ce with CpH, here both cyclopentadiene molecules are deprotonated by the basic NHC ligands resulting in two cyclopentadienyl and two imidazolium groups. One of the cyclopentadienyl ligands is trapped at the metal centre leading to two η5-cyclopentadienyl ligands and two pendant imidazolium groups at the yttrium centre in a slightly distorted tetrahedral environment (average angle is 109.1°). Most interestingly, the second cyclopentadienyl unit is subsequently entrapped by two imidazolium fragments, bonding from a neighbouring molecule through Ccarbene–H⋯(π-Cp) hydrogen bonds, giving rise to a polymeric chain (Scheme 5). Interestingly, neither of the homoleptic complexes 3 and 4 react with cyclopentadiene. Furthermore, the reactions of all homoleptic and heteroleptic compounds described in this manuscript with indene or fluorene do not lead to any products, which might be a consequence of the less acidic nature of these substrates (pKa (indene) = 20.1, pKa (fluorene) = 22.6).
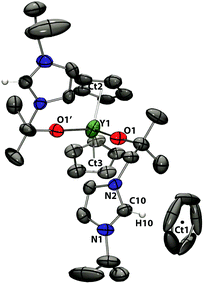 | ||
| Fig. 8 Solid state structure of 9 (asymmetric unit shown). For clarity, H atoms, except the imidazolium proton, are omitted (displacement ellipsoids drawn at 50% probability). | ||
The solid state structure of 8a contains a four coordinate cerium cation in a slightly distorted tetrahedral environment which is surrounded by three cyclopentadienyl ligands and the coordinated alkoxide arm of a protonated NHC imidazolium ligand (average tetrahedral angle = 109.02°). The Ce–CpCt bond distances of 2.633 Å are slightly elongated when compared to 1-Ce (2.594 Å), CeCp3 (2.548 Å)55 or the ketone adducts CeCp3(Ph2CO)56 and CeCp3(C13H8O).56 The retention of the Ce(III) oxidation state is supported by the Ce–O bond length, which is in good agreement with other Ce(III)–O distances and the Ce–CpCt bond distances which are significantly longer to those found in Ce(IV)–Cp compounds, e.g. CeCp3Cl.57
In 9, the hydrogen-bonded cyclopentadienyl anion is disordered due to its free rotation. The average C–C bond distance of the imidazolium-trapped cyclopentadienyl anion in 9 is 1.326 Å and the average C–C–C bond angle is 108.0° which is comparable to those found in the closely related compound [Imid2Cp][Cp2YbCl2]58 and the “free” [C5H5]− anion.59 The Ccarbene–CpCt distance is 3.214 Å and therefore slightly elongated compared to those in [Imid2Cp][Cp2YbCl2] 3.086(8) Å, but still significantly shorter than, for example, in the neutral 4-methylpyridine hexamer (3.85 Å).60 The CpCt⋯H bond distance is 2.227 Å and almost identical to those seen in [Imid2Cp][Cp2YbCl2] (2.295(9) Å) and consequently at the short end of the range of C–H⋯CpCt contacts. The Ccarbene–H–CpCt and H–CpCt–H angles are 173.61 and 178.71°, respectively. The two imidazolium ligands are arranged at an angle of 66° with respect to each other. Due to the polymeric nature of 9, the compound is virtually insoluble in aromatic and aliphatic solvents after initial crystallisation and decomposes rapidly in pyridine or chlorinated solvents, preventing detailed NMR spectroscopic analysis.
C–H acidic substrates – diphenylacetone
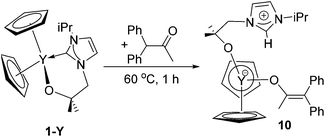
The ketone 1,1-diphenylacetone is a highly reactive substrate for electropositive metals; the pKa of the most acidic α-H is 19.4, close to that for indene, but the deprotonated molecule can coordinate to a metal centre in the enolate form. Accordingly, the reaction between 1-Y and one equivalent of Ph2CHCOCH3 in thf at 60 °C for one hour results in the formation of the zwitterionic yttrium imidazolium enolate YCp2(LH)(O–C(Me)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2) 10 (Scheme 6). A similar reaction at room temperature results in a different complex 10a which we have not yet been able to characterise structurally, but which is cleanly converted into 10 upon heating to 60 °C; we assume that 10a is a kinetic isomer of 10.
CPh2) 10 (Scheme 6). A similar reaction at room temperature results in a different complex 10a which we have not yet been able to characterise structurally, but which is cleanly converted into 10 upon heating to 60 °C; we assume that 10a is a kinetic isomer of 10.
The 1H NMR spectrum of 10 in d8-thf contains a single resonance for both cyclopentadienyl ligands at 5.90 ppm. The characteristic resonance for imidazolium proton is present at 8.86 ppm and thus significantly shifted to higher frequency when compared to 5 or 6; a 1H/13C HSQC spectrum allows the unambiguous assignment of the imidazolium proton. Besides the aromatic signals corresponding to the enolate, the spectrum also contains the other resonances of the alkoxy-tethered carbene that are slightly shifted when compared to the starting material 1-Y. The 13C{1H} NMR spectrum of 10 in d8-thf includes a singlet corresponding to the protonated imidazolium carbon atom at 138.8 ppm, and a doublet at 160.9 ppm and a singlet at 111.2 ppm which can be assigned to the two enolate alkene carbon environments.
Single crystals of 10 suitable for X-ray crystallography were grown from a concentrated thf solution at room temperature (Fig. 9). The yttrium centre in 10 is coordinated in a distorted tetrahedral coordination environment (average angle 111.3°) by two cyclopentadienyl ligands, a pendant alkoxy-tethered imidazolium ligand and the diphenylenolate group. The geometrical data for the two cyclopentadienyl ligands and the pendant ligand in 10 are almost identical to those observed for 5 and 6. The Y–O bond length of the coordinated enolate is 2.149(5) Å, slightly longer than the corresponding Y–O length of the alkoxy tether present in the same molecule, but both Y–O distances fall within the expected range. The C21–C22 distance in the enolate is 1.373(11) Å and thus slightly elongated when compared to the related compounds ZrCp2(Me)(O–C(Me)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2) (1.337(8) Å) and ZrCp2(O–C(Me)
CPh2) (1.337(8) Å) and ZrCp2(O–C(Me)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh2)2 (1.34(1) and 1.33(1) Å).61
CPh2)2 (1.34(1) and 1.33(1) Å).61
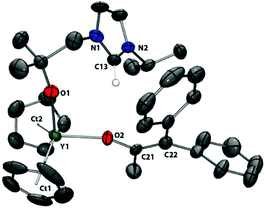 | ||
| Fig. 9 Solid state structure of 10. For clarity, H atoms, except the imidazolium proton, are omitted (displacement ellipsoids drawn at 50% probability). | ||
Conclusions
N–H-cleavable substrates pKa 21–23 (pyrrole and indole)
Complex 1-Y is very reactive towards the heterolytic cleavage of the N–H bond of pyrrole and indole across the metal–carbene bond. Control reactions show that it is the NH and not the ortho C–H bond that is cleaved in a kinetically and thermodynamically preferred step. The fact that 1-Ce does not react with these substrates could be due to the presence of THF, which is a sufficiently strongly coordinating ligand that prevents access to this larger metal cation (the Y complex never coordinates THF), or that the Ce cation is insufficiently Lewis acidic to engender heterolytic N–H cleavage across the Ce–C bond. The homoleptic alkoxy-tethered NHC complexes 3 and 4 that have many (3 or 4) coordinated alkoxide groups are presumably insufficiently Lewis acidic at the metal centre to cleave the N–H bond, but still capable of forming hydrogen-bonded adducts with the substrates.C–H-cleavable substrates pKa 25–29 (alkyne); 18 (cyclopentadiene); 20–22 (indene and fluorene); 19 (1,1′-diphenylacetone)
Complexes 1 (Y and Sc) which were reactive towards N–H cleavage do not form products of C–H cleavage even with the highly acidic C–H substrate CpH, although there appears to be a dynamic equilibrium process occurring, presumably involving a reversible C–H addition process, since the CpH substrate does not dimerise under these reaction conditions. The mono(carbene) and bis(carbene) complexes 1 and 2, respectively, both react with CpH to form zwitterionic metal cyclopentadienyl complexes that incorporate protonated imidazolium groups. In 2, due to the presence of two Y–C(NHC) bonds, this results in a polymeric complex with the very rare H+–[C5H5]–H+ motif, previously only observed in [Imid2Cp][Cp2YbCl2].58 It is notable that the other carbocycles indene and fluorene do not react, despite their high carbon C–H acidity. The substrates with less acidic E–H bonds that can form a thermodynamically stronger Ln–E bond, in particular Ln–N (5 and 6 from pyrrole and indole respectively) and Ln–O (10 from diphenylacetone), readily form M–NHC addition products.Finally, the observation that a reaction of 1-Ce with alkynes occurs (albeit to rearrange to the known Cp adduct and polymerised alkynide materials), suggests that the strength of the Ce–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CR bond provides an excellent driving force for these reactions. Work is in progress to isolate the alkynide adducts and to pursue secondary substrate insertion chemistry of these activated substrates that could lead to useful functionalised products and catalytic chemical cycles.
CR bond provides an excellent driving force for these reactions. Work is in progress to isolate the alkynide adducts and to pursue secondary substrate insertion chemistry of these activated substrates that could lead to useful functionalised products and catalytic chemical cycles.
Experimental
General procedures
All manipulations were carried out under a dry, oxygen-free dinitrogen atmosphere using standard Schlenk techniques or in a glovebox unless otherwise stated. Solvents (toluene, hexane and THF) were dried by passage activated 4 Å molecular sieve towers, stored over activated 4 Å molecular sieves and degassed three times prior to use. Deuterated solvents were refluxed over potassium, vacuum transferred and freeze–pump–thaw degassed three times prior to use. LnCp3 (Ln = Sc, Y, Ce),62 compound 332 and KL63 were synthesised according to literature procedures. All other reagents were used as purchased. 1H NMR spectra were recorded at 298 K unless otherwise stated on a Bruker AVA500 at 500 MHz. 13C and 13C{1H} NMR spectra were recorded at 298 K on a Bruker AVA500 at 125.77 MHz. The 1H, 13C and 13C{1H} NMR spectra were referenced internally to residual protio solvent (1H) or solvent (13C) and are reported to tetramethylsilane (δ = 0 ppm). Chemical shifts are quoted in δ (ppm) and coupling constants in Hz. Elemental analyses were determined by Mr Stephen Boyer at London Metropolitan University and Medac Ltd. Crystallographic data were collected on an Oxford Diffraction Xcalibur diffractometer using graphite-monochromated Mo Kα radiation (λ = 0.71073 Å). Cif files were deposited with the CCDC, codes 1003236–1003246.1-Sc – Sc(Cp)3 (0.300 g, 1.25 mmol) and KL (0.274 g, 1.25 mmol) were combined in an ampoule and thf (20 mL) added. The reaction mixture was heated to reflux for 2 hours to afford a yellow solution. Upon allowing to cool to room temperature, colourless crystals of KCp formed. The solution was decanted away from the KCp into a Schlenk and the yellow solution of product 1-Sc was concentrated to the point of crystallisation, then cooled to −30 °C. Yellow crystals of 1-Sc were isolated by filtration and dried under reduced pressure. Yield: 0.210 g, 47%.
1H NMR (C6D6, 400 MHz): 6.36 (s, br, 1H, NCHCHN), 6.13 (s, br, 1H, NCHCHN), 6.12 (s, 10H, C5H5), 4.22 (sept, 3JHH = 6.8 Hz, 1H, CHMe2), 3.24 (s, 2H, CH2CMe2O), 1.09 (s, 6H, CH2CMe2O), 0.99 (d, J = 6.7 Hz, 6H, CHMe2); Anal. Found (Calcd for C20H27N2O1Sc): C, 67.40 (67.26) H, 7.64 (7.57), N, 7.86 (7.72).
1-Y – Following a similar procedure for 1-Sc, to a suspension of YCp3 (0.30 g, 1.06 mmol) in thf (10 mL) were added KL (0.230 g, 1.05 mmol) in thf (10 mL), and the resulting reaction mixture was heated to reflux for 30 min to afford a yellow solution. Upon allowing to cool to room temperature, colourless crystals of KCp formed. The solution was decanted away from the KCp into a Schlenk and the solvent was removed under reduced pressure until colourless crystals of 1-Y began to form, then cooled to −30 °C. Colourless crystalline 1-Y was isolated by filtration and dried under reduced pressure. Yield: 0.316 g, 78%.
1H NMR (C6D6, 400 MHz): 6.20 (s, 10H, C5H5), 6.17 (d, 3JHH = 1.6 Hz, 1H, NCHCHN), 6.14 (d, 3JHH = 1.6 Hz, 1H, NCHCHN), 4.18 (sept, 3JHH = 6.8 Hz, 1H, CHMe2), 3.34 (s, 2H, CH2CMe2O), 1.09 (s, 6H, CH2CMe2O), 1.01 (d, J = 6.8 Hz, 6H, CHMe2).
13C NMR (C6D6, 126 MHz): 192.06 (d, 1JYC = 47.6 Hz, NCN), 124.00 (s, NCHCHN), 113.82 (s, NCHCHN), 109.49 (s, C5H5) 72.26 (d, J = 3.7 Hz, CMe2), 65.62 (s, OCMe2CH2), 52.80 (s, CHMe2), 29.08 (s, CH2CMe2O), 23.37 (s, CHMe2); 1H NMR (d8-thf, 500 MHz): 7.22 (d, 3JHH = 1.7 Hz, 1H, NCHCHN), 7.06 (d, 3JHH = 1.6 Hz, 1H, NCHCHN), 5.91 (s, 10H, C5H5), 4.54 (sept, 3JHH = 6.7 Hz, 1H, CHMe2), 3.69 (s, 2H, CH2CMe2O), 1.56 (d, J = 6.7 Hz, 6H, CHMe2), 0.93 (s, 6H, CH2CMe2O); 13C NMR (d8-thf, 126 MHz): 192.99 (d, 1JYC = 46.1 Hz, NCN), 125.22 (s, NCHCHN), 115.42 (s, NCHCHN), 109.34 (s, C5H5) 72.60 (d, J = 3.6 Hz, CMe2), 66.03 (s, OCMe2CH2), 53.71 (s, CHMe2), 29.38 (s, CH2CMe2O), 24.05 (s, CHMe2); Anal. Found (Calcd for C20H27N2O1Y): C, 60.21 (60.00), H, 6.99 (6.80), N, 7.27 (7.00).
1-Ce was prepared according to the method described above for the synthesis of 1-Y. It should be noted that the coordinated thf molecule observed in the solid-state structure is lost upon drying under vacuum. Yield: 0.342 g, 75%.
Anal. Found (Calcd for C20H27N2O1Ce): C, 52.99 (53.20), H, 6.00 (6.03), N, 6.21 (6.20).
2-Y – Following the same procedure mentioned above for the synthesis of 1-Y, to a suspension of YCp3 (0.30 g, 1.06 mmol) in thf (10 mL) was added KL (0.460 g, 2.10 mmol) in thf (15 mL), and the resulting reaction mixture was heated to reflux for 45 min to afford a yellow solution. Upon allowing to cool to room temperature, colourless crystals of KCp formed. The solution was decanted away from the KCp into a Schlenk and the solvent was slowly removed under reduced pressure until colourless crystals of 2 began to form. Further crystals of 2 were afforded by cooling to −30 °C. These crystals were isolated by filtration and dried under reduced pressure. Yield: 0.381 g, 71%.
1H NMR (C6D6, 500 MHz): 6.53 (s, 5H, C5H5), 6.37 (d, 3JHH = 1.6 Hz, 2H, NCHCHN), 6.20 (d, 3JHH = 1.6 Hz, 2H, NCHCHN), 5.73 (sept, 3JHH = 6.8 Hz, 2H, CHMe2), 3.82 (d, 3JHH = 12.5 Hz, 2H, CH2CMe2O), 3.30 (d, 3JHH = 12.5 Hz, 2H, CH2CMe2O), 1.31 (s, 6H, CH2CMe2O), 1.28 (d, J = 6.8 Hz, 6H, CHMe2), 1.26 (d, J = 6.8 Hz, 6H, CHMe2), 0.77 (s, 6H, CH2CMe2O); 13C NMR (C6D6, 126 MHz): 194.33 (d, 1JYC = 37.3 Hz, NCN), 121.97 (s, NCHCHN), 113.14 (s, NCHCHN), 108.69 (s, C5H5) 71.10 (d, J = 2.9 Hz, CMe2), 65.30 (s, OCMe2CH2), 50.76 (s, CHMe2), 31.22 (s, CH2CMe2O), 28.84 (s, CH2CMe2O), 24.36 (s, CHMe2), 23.39 (s, CHMe2); 1H NMR (d8-thf, 500 MHz): 7.02 (d, 3JHH = 1.6 Hz, 2H, NCHCHN), 6.88 (d, 3JHH = 1.6 Hz, 2H, NCHCHN), 6.00 (s, 5H, C5H5), 5.63 (sept, 3JHH = 6.8 Hz, 2H, CHMe2), 3.80 (d, 3JHH = 12.4 Hz, 2H, CH2CMe2O), 3.70 (d, 3JHH = 12.4 Hz, 2H, CH2CMe2O), 1.45 (d, J = 6.8 Hz, 6H, CHMe2), 1.40 (d, J = 6.8 Hz, 6H, CHMe2), 1.15 (s, 6H, CH2CMe2O), 0.60 (s, 6H, CH2CMe2O); 13C NMR (d8-thf, 101 MHz): 194.79 (d, 1JYC = 37.5 Hz, NCN), 122.99 (s, NCHCHN), 114.25 (s, NCHCHN), 108.58 (s, C5H5), 71.72 (d, J = 2.9 Hz, CMe2), 65.84 (s, OCMe2CH2), 51.47 (s, CHMe2), 31.39 (s, CH2CMe2O), 29.00 (s, CH2CMe2O), 24.66 (s, CHMe2), 23.68 (s, CHMe2); Anal. Found (Calcd for C25H39N4O2Y): C, 58.00 (58.13), H, 7.29 (7.61), N, 10.51 (10.85).
4 – KL (500 mg, 2.27 mmol, 4 eq.) and ThCl4(dme)2 (320 mg, 0.58 mmol, 1 eq.) were dissolved in thf and stirred for ca. 1 h before the solvent was removed under reduced pressure. Toluene was added and the suspension was filtered and washed with toluene. The solvent was then half removed in vacuum and the resulting solution cooled to −30 °C yielding colourless crystals (455 mg, 82%). 1H NMR (303 K, C6D6) δ 6.53 (s, 4H, CH), 6.38 (s, 4 h, CH), 6.02 (br. S, 4H, CH(CH3)2), 3.44 (br. S, 8H, CH2), 1.30 (br s., 48H, C(CH3)2 and CH(CH3)3) ppm; 1H NMR (343 K, C6D6) δ 6.56 (s, 4H, CH), 6.48 (s, 4H, CH), 5.88 (sept., J = 6.46 Hz, 4H, CH(CH3)2), 3.81 (s, 8H, CH2), 1.27 (d, J = 6.45 Hz, 24H, CH(CH3)2), 1.13 (s, 24H, C(CH3)2) ppm; 1H NMR (213 K, THF–C6D6) δ 6.85 (s, 2H, CH), 6.71 (s, 2H, CH), 6.62 (s, 2H, CH), 6.49 (s, 2H, CH), 6.10 (sept., J = 6.46 Hz, 2H, CH(CH3)2), 5.71 (sept., J = 6.46 Hz, 2H, CH(CH3)2), 4.79 (d, J = 11.55 Hz, 2H, CH2), 3.7–3.2 (missing doublet under THF, 2H), 3.18 (d, J = 11.55 Hz, 2H, CH2), 3.03 (d, J = 11.55 Hz, 2H, CH2), 1.75–1.25 (two missing singlets under THF, 24H), 1.23 (d, J = 6.45 Hz, 6H, CH(CH3)2), 0.48 (d, J = 6.45 Hz, 12H, CH(CH3)2), 0.36 (d, J = 6.45 Hz, 6H, CH(CH3)2) ppm; 13C (303 K, C6D6) δ 210.3 (NCN), 121.8 (CH), 113.1 (CH), 75.21 (CH2), 64.3 (C(CH3)2), 51.2 (CH(CH3)2), 29.1 (CH3) 24.0 (CH3). Anal. Found (Calcd for C40H72N8O4Th): C, 46.59 (49.99), H, 9.32 (7.55), N, 10.07 (11.66).
5 – To a solution of freshly prepared 1-Y (0.20 g, 0.50 mmol) in thf (5 mL) was added pyrrole (0.034 g, 0.50 mmol) and the resulting mixture was stirred at room temperature for 1 h, during which time a colourless precipitate formed. The precipitate was collected by filtration, washed with hexanes (3 × 3 mL) and dried under vacuum to afford 3 as a colourless material. Yield: 0.159 g (68%). Diffraction-quality crystals were grown from a concentrated thf solution at room temperature.
1H NMR (d8-thf, 500 MHz): 7.38 (d, 3JHH = 1.9 Hz, 1H, NCHCHN), 7.12 (d, 3JHH = 1.9 Hz, 1H, NCHCHN), 6.86 (t, 3JHH = 1.6 Hz, 2H, NCHCH), 6.32 (s, br, HCNN), 5.99 (t, 3JHH = 1.6 Hz, 2H, NCHCH), 5.89 (s, 10H, C5H5), 4.46 (sept, 3JHH = 6.7 Hz, 1H, CHMe2), 3.74 (s, 2H, CH2CMe2O), 1.37 (d, J = 6.7 Hz, 6H, CH, 1.07 (s, 6H, CH2CMe2O); 13C NMR (d8-thf, 126 MHz): 138.8 (s, NCHN), 129.2 (s, pyrrole CH), 125.1 (s, NCHCHN), 118.1 (NCHCHN), 109.5 (s, C5H5), 107.24 (s, pyrrole CH), 72.2 (d, J = 5.8 Hz, CMe2), 63.3 (CH2CMe2O), 53.5 (HCMe2), 30.1 (CH2CMe2O), 22.9 (CHMe2) ppm; Anal. Found (Calcd for C24H32N3O1Y): C, 62.10 (61.67), H, 6.96 (6.90), N, 9.12 (8.99).
6 – Following the procedure for 3, to a solution of freshly prepared 1-Y (0.20 g, 0.50 mmol) in thf (5 mL) was added indole (0.059 g, 0.50 mmol) and the resulting mixture was stirred at room temperature for 1 h, during which time a colourless precipitate formed. The precipitate was collected by filtration, washed with hexanes (3 × 3 mL) and dried in vacuo to afford 4 as a colourless material. Yield: 0.183 g (71%). Diffraction-quality crystals were grown from a concentrated thf solution at room temperature.
1H NMR (d8-thf, 500 MHz): 7.64 (d, 3JHH = 8.1 Hz, 1H, NC8H6), 7.48 (d, 3JHH = 2.5 Hz, 1H, NC8H6), 7.42 (d, 3JHH = 7.7 Hz, 1H, NC8H6), 7.37 (s, br, HCNN), 7.27 (s, br, 1H, NCHCHN), 7.22 (s, br, 1H, NCHCHN), 6.81 (dt, 6.5 Hz, 1.0 Hz, NC8H6), 6.72 (dt, 6.5 Hz, 1.0 Hz, NC8H6), 6.36 (d, 3JHH = 2.5 Hz, 1H, NC8H6), 5.92 (s, 10H, C5H5), 3.83 (s, 2H, CH2CMe2O), 3.24 (sept, 3JHH = 6.7 Hz, 1H, CHMe2), 1.22 (s, 6H, CH2CMe2O) 0.96 (d, J = 6.7 Hz, 6H, CHMe2); 13C NMR (d8-thf, 126 MHz): 147.9 (s, indole C), 138.9 (s, indole, CH), 137.2 (s, NCHN), 132.3 (s, indole C), 124.8 (s, NCHCHN), 119.6 (s, indole CH), 118.3 (s, NCHCHN), 118.0 (s, indole CH), 117.5 (s, indole CH), 117.0 (s, indole CH), 109.6 (s, C5H5), 71.4 (d, J = 5.5 Hz, CMe2), 62.8 (s, CH2CMe2O), 52.3 (s, HCMe2), 30.3 (s, CH2CMe2O), 22.5 (s, CHMe2) ppm; Anal. Found (Calcd for C28H34N3O1Y): C, 65.32 (64.99), H, 6.28 (6.62), N, 8.13 (8.12).
7–3 (0.068 g, 0.11 mmol) and pyrrole (0.008 g, 0.11 mmol) were combined in benzene (2 mL) resulting in a yellow solution. Hexane was slowly diffused into the reaction mixture affording large yellow crystals of 6. 1H NMR (C6D6, 500 MHz, 298 K): 11.61 (br s, 1H, NH), 7.06 (br s, 2H, pyrrole CH), 6.43 (br s, 2H, pyrrole CH), 6.34 (s, 3H, CHMe2), 6.30 (s, 3H, NCHCHN), 5.69 (s, 3H, CHMe2), 3.96 (s, 6H, CH2CMe2O), 1.24 (br s, 18H, CH2CMe2O), 0.97 (br s, 18H, CHMe2) ppm. 13C NMR (C6D6, 126 MHz): 194.7 (NCN), 130.2 9 (pyrrole CH), 121.8 (NCHCHN), 119.8 (pyrrole CH), 112.6 (NCHCHCN), 107.4 (pyrrole CH), 104.19 (pyrrole CH), 72.0 (CH2CMe2O), 63.9 (CH2CMe2O), 50.2 (HCMe2), 30.6 (CH2CMe2O), 23.4 (CHMe2) ppm. Yield: Quantitative by 1H NMR spectroscopy. No microanalysis was determined due to loss of pyrrole on drying under vacuum.
Reactivity of 4 towards pyrrole
Neat pyrrole (3.5 mg, 0.05 mmol) was added to a solution of 4 (0.05 g, 0.05 mmol) in C5D5N in a Teflon-valved NMR tube, resulting in a pale orange solution. 1H NMR (500 MHz, C5D5N, 298 K): δ 10.04 (br. s, 1H), 7.48 (s, 2H), 7.09 (s, 8H), 6.67 (s, 2H), 5.75 (br. s, 4H), 3.98 (br. s, 8H), 1.37 (br. s, 24H), 1.16 (br. s, 24H) ppm.In a similar manner, an excess of pyrrole (0.05 g, 0.75 mmol) was added to a solution of 4 (0.02 g, 0.02 mmol) in C5D5N in a Teflon-valved NMR tube, resulting in a pale orange solution. 1H NMR (500 MHz, C5D5N, 298 K) δ 11.23 (s, 7H), 7.50 (s, 1H), 7.46 (s, 1H), 7.44 (s, 1H), 7.41 (s, 1H), 7.36 (s, 1H), 7.20 (t, J = 1.9 Hz, 10H), 7.12 (s, 1H), 6.61 (s, 1H), 6.52 (t, J = 1.9 Hz, 10H), 6.39 (s, 1H), 4.53 (s, 1H), 4.45 (s, 1H), 4.10 (s, 2H), 4.07 (s, 2H), 3.99 (s, 4H), 1.41 (s, 6H), 1.36 (s, 21H), 1.25 (s, 12H) ppm.
8a – To a solution of freshly prepared 1-Ce (0.30 g, 0.66 mmol) in thf (5 mL) was added freshly distilled cyclopentadiene (0.044 g, 0.66 mmol) and the resulting mixture was stirred at 60 °C for 4 h during which time a colourless precipitate formed. The precipitate was collected by filtration, washed with hexanes (3 × 3 mL) and dried under vacuum to afford 8a as a colourless material. Yield: 0.219 g (64%). Diffraction-quality crystals were grown from a concentrated thf solution. Poor solubility of the product prevented NMR analyses.
Anal. Found (Calcd for C25H33N2O1Ce): C, 57.58 (58.01), H, 6.01 (6.43), N, 5.53 (5.41).
9 – To a solution of freshly prepared 2-Y (0.30 g, 0.58 mmol) in thf (5 mL) was added freshly distilled cyclopentadiene (0.039 g, 0.58 mmol) and the resulting mixture was stirred at 60 °C for 4 h during which time a colourless precipitate formed. The precipitate was collected by filtration, washed with hexanes (3 × 3 mL) and dried under vacuum to afford 9 as colourless crystals. Yield: 0.256 g (68%). Diffraction-quality crystals were grown from a concentrated thf solution. Poor solubility of the product prevented NMR analyses.
Anal. Found (Calcd for C35H51N4O2Y): C, 64.44 (64.80), H, 7.54 (7.92), N, 8.22 (8.64).
10 – To a solution of freshly prepared 1-Y (0.20 g, 0.50 mmol) in thf (5 mL) was added 1,1-diphenylacetone (0.105 g, 0.5 mmol) and the resulting mixture was stirred at 60 °C for 1 h, during which time a colourless precipitate formed. The precipitate was collected by filtration, washed with hexanes (3 × 3 mL) and dried under vacuum to afford 10 as a colourless material. Yield: 0.192 g (63%). Diffraction-quality crystals were grown from a concentrated thf solution at room temperature.
1H NMR (d8-thf, 500 MHz): 8.86 (s, HCNN), 7.48 (d, 3JHH = 7.4 Hz, 2H, (C6H5)2CCOMe), 7.29 (s, br, 1H, NCHCHN), 7.21 (t, 3JHH = 7.6 Hz, 2H, (C6H5)2CCOMe), 7.15–7.09 (m, 3H), 7.08–7.01 (m, 3H), 6.78 (t, 3JHH = 7.3 Hz, 1H, (C6H5)2CCOMe), 5.90 (s, 10H, C5H5), 4.56 (sept, 3JHH = 6.7 Hz, 1H, CHMe2), 3.48 (s, 2H, CH2CMe2O), 1.98 (s, 3H, (C6H5)2CCOMe), 1.51 (d, J = 6.7 Hz, 6H, CHMe2), 1.06 (s, 6H, CH2CMe2O); 13C NMR (d8-thf, 101 MHz): 160.9 (d, 2JYC = 3.3 Hz, (C6H5)2CCOMe), 147.1 (s, ipso-(C6H5)2CCOMe)), 146.0 (s, ipso-(C6H5)2CCOMe)), 138.8 (s, NCHN), 132.9 (s, (C6H5)2CCOMe), 131.1 (s, (C6H5)2CCOMe), 128.5 (s, (C6H5)2CCOMe), 128.0 (s, (C6H5)2CCOMe), 125.1 (s, (C6H5)2CCOMe), 124.5 (s, NCHCHN), 123.5 (s, (C6H5)2CCOMe), 117.7 (s, NCHCHN), 111.2 (s, (C6H5)2CCOMe), 109.3 (s, C5H5) 72.7 (d, J = 5.7 Hz, CMe2), 63.2 (s, OCMe2CH2), 53.5 (s, CHMe2), 30.0 (s, CH2CMe2O), 26.0 (s, (C6H5)2CCOMe), 23.3 (s, CHMe2) ppm; Anal. Found (Calcd for C35H41N2O2Y): C, 68.61 (68.84), H, 6.56 (6.77), N, 4.45 (4.59).
10a – An isomer of 10, labelled 10a can also be isolated from the room-temperature reaction between 1,1-diphenylacetone and 1-Y.
To a solution of freshly prepared 1-Y (0.20 g, 0.50 mmol) in thf (5 mL) was added 1,1-diphenylacetone (0.105 g, 0.5 mmol) and the resulting mixture was stirred at room temperature for 1 h. The resulting solution was cooled to −30 °C upon which large colourless crystals formed. The crystals were collected by filtration, washed with hexanes (3 × 3 mL) and dried under vacuum to afford 10a as a colourless material. Yield: 0.125 g (41%). Crystals grown from a concentrated thf solution at −30 °C were poorly diffracting. It should be noted that 10a can be converted quantitatively into 10 by heating a thf solution of 10a for 1 h at 60 °C.
1H NMR (d8-thf, 500 MHz): 9.12 (s, HCNN), 7.44 (d, 3JHH = 7.4 Hz, 2H, (C6H5)2CCOMe), 7.31–7.17 (m, 5H), 7.15–7.03 (m, 5H), 5.78 (s, 10H, C5H5), 4.47 (sept, 3JHH = 6.7 Hz, 1H, CHMe2), 3.56 (s, 2H, CH2CMe2O), 1.98 (s, 3H, (C6H5)2CCOMe), 1.43 (d, J = 6.7 Hz, 6H, CHMe2), 0.98 (s, 6H, CH2CMe2O); 13C NMR (d8-thf, 101 MHz): 171.3 (d, 2JYC = 3.3 Hz, (C6H5)2CCOMe), 146.0 (s, ipso-(C6H5)2CCOMe)), 145.5 (s, ipso-(C6H5)2CCOMe)), 138.2 (s, NCHN), 130.6 (s, (C6H5)2CCOMe), 128.6 (s, (C6H5)2CCOMe), 128.0 (s, (C6H5)2CCOMe), 126.5 (s, (C6H5)2CCOMe), 125.5 (s, (C6H5)2CCOMe), 125.2 (s, NCHCHN), 124.5 (s, (C6H5)2CCOMe), 117.6 (s, NCHCHN), 109.4 (s, (C6H5)2CCOMe), 109.2 (s, C5H5) 71.8 (d, J = 5.7 Hz, CMe2), 63.8 (s, OCMe2CH2), 53.4 (s, CHMe2), 29.8 (s, CH2CMe2O), 26.0 (s, (C6H5)2CCOMe), 23.3 (s, CHMe2) ppm.
Acknowledgements
The authors are grateful for the support of the Technische Universität München – Institute for Advanced Study, funded by the German Excellence Initiative, Sasol Technology, the University of Edinburgh, and the EPSRC.References
- X. Bugaut and F. Glorius, Chem. Soc. Rev., 2012, 41, 3511–3522 RSC.
- V. Nair, S. Vellalath and B. P. Babu, Chem. Soc. Rev., 2008, 37, 2691–2698 RSC.
- D. Enders, O. Niemeier and A. Henseler, Chem. Rev., 2007, 107, 5606–5655 CrossRef CAS PubMed.
- S. Gaillard, C. S. J. Cazin and S. P. Nolan, Acc. Chem. Res., 2012, 45, 778–787 CrossRef CAS PubMed.
- F. Glorius, N-Heterocyclic Carbenes in Transition Metal Catalysis, Springer, Berlin, 2007 Search PubMed.
- F. E. Hahn and M. C. Jahnke, Angew. Chem., Int. Ed., 2008, 47, 3122–3172 CrossRef CAS PubMed.
- H. Nakai, X. Hu, L. N. Zakharov, A. L. Rheingold and K. Meyer, Inorg. Chem., 2004, 43, 855–857 CrossRef CAS PubMed.
- P. L. Arnold and S. T. Liddle, Chem. Commun., 2006, 3959–3971 RSC.
- T. Mehdoui, J. C. Berthet, P. Thuery and M. Ephritikhine, Chem. Commun., 2005, 2860–2862 RSC.
- P. L. Arnold and I. J. Casely, Chem. Rev., 2009, 109, 3599–3611 CrossRef CAS PubMed.
- S. T. Liddle, I. S. Edworthy and P. L. Arnold, Chem. Soc. Rev., 2007, 1732–1744 RSC.
- D. Enders, A. Grossmann, J. Fronert and G. Raabe, Chem. Commun., 2010, 46, 6282–6284 RSC.
- Y. Shimakawa, T. Morikawa and S. Sakaguchi, Tetrahedron Lett., 2010, 51, 1786–1789 CrossRef CAS PubMed.
- D. A. DiRocco, K. M. Oberg, D. M. Dalton and T. Rovis, J. Am. Chem. Soc., 2009, 131, 10872–10874 CrossRef CAS PubMed.
- Q. Liu, S. Perreault and T. Rovis, J. Am. Chem. Soc., 2008, 130, 14066–14067 CrossRef CAS PubMed.
- R. Singh and S. P. Nolan, Chem. Commun., 2005, 5456–5458 RSC.
- V. Nair, S. Bindu and V. Sreekumar, Angew. Chem., Int. Ed., 2004, 43, 5130–5135 CrossRef CAS PubMed.
- G. A. Grasa, T. Gueveli, R. Singh and S. P. Nolan, J. Org. Chem., 2003, 68, 2812–2819 CrossRef CAS PubMed.
- A. Grossmann and D. Enders, Angew. Chem., Int. Ed., 2012, 51, 314–325 CrossRef CAS PubMed.
- L. Candish and D. W. Lupton, Chem. Sci., 2012, 3, 380–383 RSC.
- T. Kano, K. Sasaki and K. Maruoka, Org. Lett., 2005, 7, 1347–1349 CrossRef CAS PubMed.
- S. Diez-Gonzalez, N. Marion and S. P. Nolan, Chem. Rev., 2009, 109, 3612–3676 CrossRef CAS PubMed.
- P. L. Watson and G. W. Parshall, Acc. Chem. Res., 1985, 18, 51–56 CrossRef CAS.
- B. A. Vastine and M. B. Hall, Coord. Chem. Rev., 2009, 253, 1202–1218 CrossRef CAS PubMed.
- W. J. Evans, Inorg. Chem., 2007, 46, 3435–3449 CrossRef CAS PubMed.
- T. E. Mueller, K. C. Hultzsch, M. Yus, F. Foubelo and M. Tada, Chem. Rev., 2008, 108, 3795–3892 CrossRef CAS PubMed.
- X. Yu, S. Seo and T. J. Marks, J. Am. Chem. Soc., 2007, 129, 7244–7245 CrossRef CAS PubMed.
- S. Hong and T. J. Marks, Acc. Chem. Res., 2004, 37, 673–686 CrossRef CAS PubMed.
- B. Liu and D. Cui, Dalton Trans., 2009, 550–556 RSC.
- B. D. Stubbert and T. J. Marks, J. Am. Chem. Soc., 2007, 129, 4253–4271 CrossRef CAS PubMed.
- S. Seo, X. Yu and T. J. Marks, J. Am. Chem. Soc., 2009, 131, 263–276 CrossRef CAS PubMed.
- P. L. Arnold, I. A. Marr, S. Zlatogorsky, R. Bellabarba and R. P. Tooze, Dalton Trans., 2014, 43, 34–37 RSC.
- P. L. Arnold, Z. R. Turner, A. I. Germeroth, I. J. Casely, G. S. Nichol, R. Bellabarba and R. P. Tooze, Dalton Trans., 2013, 42, 1333–1337 RSC.
- Z. R. Turner, R. Bellabarba, R. P. Tooze and P. L. Arnold, J. Am. Chem. Soc., 2010, 132, 4050–4051 CrossRef CAS PubMed.
- P. L. Arnold, Z. R. Turner, R. Bellabarba and R. P. Tooze, J. Am. Chem. Soc., 2011, 133, 11744–11756 CrossRef CAS PubMed.
- J. A. Joule and K. Mills, Heterocyclic Chemistry, Blackwell Science, Oxford, 4th edn, 2000 Search PubMed.
- M. Ganesan, M. P. Lalonde, S. Gambarotta and G. P. A. Yap, Organometallics, 2001, 20, 2443–2445 CrossRef CAS.
- P. L. Arnold, J. H. Farnaby, R. C. White, N. Kaltsoyannis, M. G. Gardiner and J. B. Love, Chem. Sci., 2014, 5, 756–765 RSC.
- J. W. Leeland, F. J. White and J. B. Love, Chem. Commun., 2011, 47, 4132–4134 RSC.
- J. S. Hart, F. J. White and J. B. Love, Chem. Commun., 2011, 47, 5711–5713 RSC.
- J. B. Love, Chem. Commun., 2009, 3154–3165 RSC.
- S. Yang, X. Zhu, S. Zhou, S. Wang, Z. Feng, Y. Wei, H. Miao, L. Guo, F. Wang, G. Zhang, X. Gu and X. Mu, Dalton Trans., 2014, 43, 2521–2533 RSC.
- X. Zhu, S. Zhou, S. Wang, Y. Wei, L. Zhang, F. Wang, S. Wang and Z. Feng, Chem. Commun., 2012, 48, 12020–12022 RSC.
- Y. Yang, Q. Wang and D. Cui, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 5251–5262 CrossRef CAS.
- P. L. Arnold, S. Zlatogorsky, N. A. Jones, C. D. Carmichael, S. T. Liddle, A. J. Blake and C. Wilson, Inorg. Chem., 2008, 47, 9042–9049 CrossRef CAS PubMed.
- B. L. Wang, D. Wang, D. M. Cui, W. Gao, T. Tang, X. S. Chen and X. B. Jing, Organometallics, 2007, 26, 3167–3172 CrossRef CAS.
- S. T. Liddle and P. L. Arnold, Organometallics, 2005, 24, 2597–2605 CrossRef CAS.
- I. J. Casely, S. T. Liddle, A. J. Blake, C. Wilson and P. L. Arnold, Chem. Commun., 2007, 5037–5039 RSC.
- P. L. Arnold, A. J. Blake and C. Wilson, Chem. – Eur. J., 2005, 11, 6095–6099 CrossRef CAS PubMed.
- B. A. Belinka Jr., A. Hassner and J. M. Hendler, J. Org. Chem., 1981, 46, 631–632 CrossRef.
- S. Arndt, A. Trifonov, T. P. Spaniol, J. Okuda, M. Kitamura and T. Takahashi, J. Organomet. Chem., 2002, 647, 158–166 CrossRef CAS.
- Q. F. Mokuolu, P. A. Duckmanton, P. B. Hitchcock, C. Wilson, A. J. Blake, L. Shukla and J. B. Love, Dalton Trans., 2004, 1960–1970 RSC.
- F. G. Bordwell, G. E. Drucker, N. H. Andersen and A. D. Denniston, J. Am. Chem. Soc., 1986, 108, 7310–7313 CrossRef CAS.
- F. G. Bordwell, G. E. Drucker and H. E. Fried, J. Org. Chem., 1981, 46, 632–635 CrossRef CAS.
- U. Baisch, S. Pagano, M. Zeuner, J. Schmedt auf der Günne, O. Oeckler and W. Schnick, Organometallics, 2006, 25, 3027–3033 CrossRef CAS.
- A. R. Crozier, K. W. Törnroos, C. Maichle-Mössmer and R. Anwander, Eur. J. Inorg. Chem., 2013, 2013, 409–414 CrossRef CAS.
- P. Dröse, A. R. Crozier, S. Lashkari, J. Gottfriedsen, S. Blaurock, C. G. Hrib, C. Maichle-Mössmer, C. Schädle, R. Anwander and F. T. Edelmann, J. Am. Chem. Soc., 2010, 132, 14046–14047 CrossRef PubMed.
- C. D. Abernethy, C. L. B. Macdonald, J. A. C. Clyburne and A. H. Cowley, Chem. Commun., 2001, 61–62 RSC.
- C. D. Abernethy, J. A. C. Clyburne, A. H. Cowley and R. A. Jones, J. Am. Chem. Soc., 1999, 121, 2329–2330 CrossRef CAS.
- K. Biradha and M. J. Zaworotko, J. Am. Chem. Soc., 1998, 120, 6431–6432 CrossRef CAS.
- S. Gambarotta, S. Strologo, C. Floriani, A. Chiesi-Villa and C. Guastini, Inorg. Chem., 1985, 24, 654–660 CrossRef CAS.
- J. M. Birmingham and G. Wilkinson, J. Am. Chem. Soc., 1956, 78, 42–44 CrossRef CAS.
- P. L. Arnold, M. Rodden and C. Wilson, Chem. Commun., 2005, 1743–1745 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1003236–1003246. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4dt01442a |
| This journal is © The Royal Society of Chemistry 2014 |