Supramolecular activation of a molecular photocatalyst†
Abstract
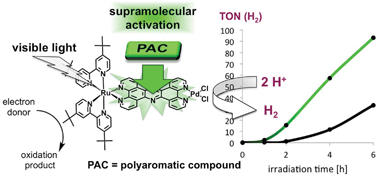
The effects of the planar aromatic organic molecules anthracene and pyrene on the catalytic performance of the intramolecular hydrogen evolving photocatalyst [Ru(tbbpy)2(tpphz)PdCl2](PF6)2 functioning as a photocatalytic dyad have been studied. 1H-NMR studies on [Ru(tbbpy)2(tpphz)PdCl2](PF6)2 and [Ru(tbbpy)2(tpphz)](PF6)2 show a pronounced interaction of pyrene with the ruthenium complexes due to π–π-interactions. The solid state structure of [Ru(tbbpy)2(tpphz)PdCl2]2[Mo8O24] shows a pronounced π–π-stacking of the polyaromatic ligands. In addition, dimerization constants for the complexes and association constants between the complexes and pyrene were determined. Studies on the photocatalytic hydrogen production show a decreased induction phase and increased turn over frequencies during the initial phase of the catalysis in the presence of anthracene and pyrene utilising the catalyst [Ru(tbbpy)2(tpphz)PdCl2](PF6)2 irrespective of the nature of the polycyclic aromatic hydrocarbon.

 Please wait while we load your content...
Please wait while we load your content...