C–H and C–O bond activation with a rhodium(i) β-diiminate complex†
Abstract
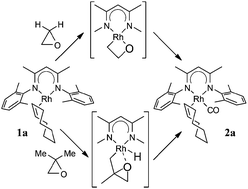
Complex LRh(COE) [L = (2,6-Me2C6H3NCMe)2CH; COE = cyclooctene] reacts with oxirane, methyloxirane and 2,2-dimethyloxirane to eventually produce LRh(COE)(CO) and alkane (methane, ethane, propane). This reaction and other aspects of the reactivity of the “LRh” fragment (hydrogenation of olefins and benzene) have been studied by density functional theory. The results indicate that for 2,2-dimethyloxirane (and probably also for methyloxirane) the reaction starts with C–H activation of a methyl group, followed by ring opening and decarbonylation. For the parent oxirane, where this path is not available, the reaction starts with C–O activation to form a 2-rhodaoxetane, which then undergoes β-elimination. More generally, easy C–H activation, which appears to be a recurring theme in this Rh chemistry, is due to a close energy matching between corresponding Rh(I) and Rh(III) complexes. For the analogous Ir complexes, preference for higher oxidation states is larger, leading to significantly higher barriers for e.g. hydrogenation.
- This article is part of the themed collection: Synergy between Experiment and Theory

 Please wait while we load your content...
Please wait while we load your content...