Syntheses, structures and properties of 5-azotetrazolyl salicylic acid and its dilanthanide complexes†
Abstract
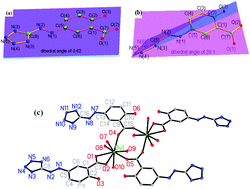
Two hydrated 5-azotetrazolyl salicylic acid (H3ASA) of [(H3ASA)·H2O] (1) and [(H3ASA)·4H2O] (2) and five H3ASA based dinuclear Ln3+ complexes of {[Ln2(H1.5ASA)4(H2O)8]·6H2O} [Ln = Dy (3), Tb(4)], {[Gd2(H1.5ASA)4 (H2O)8]·5H2O} (5), {[Sm2(H1.5ASA)4(H2O)8]·6H2O} (6) and {[Eu4(H1.5ASA)8(H2O)18]·10H2O} (7) have been synthesized and characterized by single crystal X-ray diffraction analysis. In 1 and 2, the neutral H3ASA molecules show a trans-enol-E isomer but display two different dihedral angles. Complexes 3–7 exhibit three types of dinuclear structures in which the anionic ligands show two trans-enol-E/Z isomers. The photochromic and photoluminescent properties of 1, 3–7 and the magnetic properties for 3–7 were investigated.

 Please wait while we load your content...
Please wait while we load your content...