Engineering microstructure and redox properties in the mixed conductor Ce0.9Pr0.1O2−δ + Co 2 mol%†
Abstract
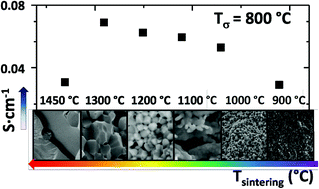
10% Praseodymium doped ceria exhibits a combination of mixed ionic and electronic conductivity, redox catalytic properties and chemical compatibility with water and carbon dioxide at high temperatures. Minor additions of cobalt oxide have been demonstrated to act as a sintering aid as well as an effective promoter of the electronic conduction. However, an excess of sintering temperature causes cobalt aggregation into the grain boundaries as inferred from FE-SEM/EDX and TEM analysis. The redox behaviour of the materials was studied by means of temperature programmed desorption (TPD) and reduction (TPR). This work shows the systematic study of sintering conditions in order to understand the evolution of the material microstructure, grain boundaries and the role of cobalt in this complex system. The final purpose of the work is to improve both electronic and oxygen ion transport properties for their potential application as oxygen-transport membranes and solid oxide fuel cell components. The sample sintered at 1000 °C exhibited the highest total conductivity at high temperatures, which is principally related to the improvement in the electronic conductivity through the grain boundary network.
- This article is part of the themed collection: New Talent: Europe

 Please wait while we load your content...
Please wait while we load your content...