The iso-VAPOL ligand: synthesis, solid-state structure and its evaluation as a BOROX catalyst†
Abstract
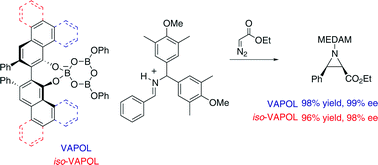
The new vaulted biaryl ligand iso-VAPOL is an isomer of VAPOL but has the chiral pocket of VANOL. The synthesis of iso-VAPOL involves a cycloaddition/electrocyclization cascade (CAEC) similar to one that is used for VAPOL except that the starting material for iso-VAPOL is less than one-tenth the cost. The solid-state structure of iso-VAPOL was determined as well as that of VANOL since it had not been previously reported. The structures of iso-VAPOL and VANOL are compared to the known solid-state structure of VAPOL and it is found that all three ligands have the cisoid conformations in the solid-state; the dihedral angle between the two aryl groups is less than 90°. In addition, all three ligands pack in the solid-state with no inter-molecular hydrogen bonds. This is the opposite to what has been reported for BINOL where all known structures exist with transoid conformations where the dihedral angle is >90° and where the BINOL units pack with hydrogen bonds between neighboring BINOL units. Spectroscopic evidence including 1H and 11B NMR spectra are presented which indicate that the iso-VAPOL ligand will form BOROX catalysts with B(OPh)3 in much the same way as VAPOL catalysts which have previously reported and characterized both by spectroscopy and X-ray crystallography. Support for the BOROX catalysts can also be taken from the fact that iso-VAPOL BOROX catalysts give essentially the same asymmetric inductions as the VAPOL BOROX catalyst over a range of substrates.
- This article is part of the themed collection: Catalysis in the USA

 Please wait while we load your content...
Please wait while we load your content...