Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Catalytic hydrotreatment of pyrolytic lignins to give alkylphenolics and aromatics using a supported Ru catalyst
Arjan
Kloekhorst
,
Jelle
Wildschut
and
Hero Jan
Heeres
*
University of Groningen, Chemical Engineering, Nijenborgh 4, 9747 AG Groningen, The Netherlands. E-mail: H.J.Heeres@rug.nl; Tel: +31(0)503634174
First published on 22nd May 2014
Abstract
The catalytic hydrotreatment of two pyrolytic lignins (pine and forestry residue), obtained from the corresponding fast pyrolysis oils, and organosolv Alcell lignin as a benchmark was explored in a batch set-up using Ru/C as the catalyst (400 °C, 4 h, 100 bar initial H2 pressure). The highest lignin oil yield was obtained for forest residue pyrolytic lignin (>75 wt% on intake). Advanced GCxGC techniques in combination with GPC and 13C-NMR measurements indicate that the lignin oils contain high amounts of interesting monomeric chemicals like alkylphenolics (up to 20.5 wt% on lignin feed intake) and aromatics (up to 14.1 wt% on lignin feed intake). These values are considerably higher than for Alcell lignin (6.6 wt% alkylphenolics and 9.7 wt% aromatics) and clearly indicate that pyrolytic lignins have potential to be used as feeds for the production of biobased phenolics and aromatics.
1 Introduction
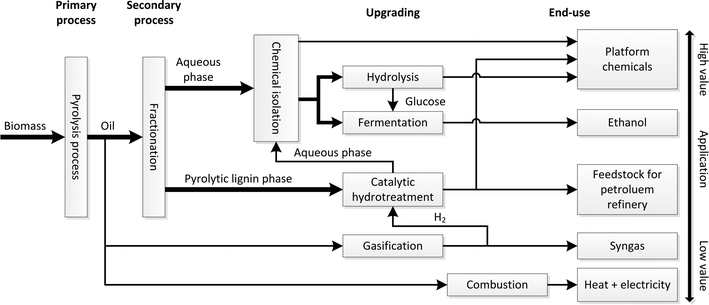
The impending depletion of fossil resources together with environmental concerns has boosted research and development activities on renewable resources. An interesting alternative is biomass, which is currently already being used for the production of carbon-based transport fuels (e.g. bioethanol, biodiesel) and has high potential for bio-based chemicals.1–4 An integrated model for biomass valorisation is the biorefinery concept, which is defined as the sustainable processing of biomass into a spectrum of marketable products and energy.5 Various biorefinery models have been described and are actively being explored at the moment.An interesting example of the biorefinery concept is a pyrolysis oil biorefinery (see Fig. 1 for details). It involves the use of pyrolysis oil as a feed for a biorefinery. Pyrolysis oil is accessible from (lignocellulosic) biomass using fast pyrolysis technology in yields up to 70 wt%. It is considered an attractive liquefied form of biomass that has considerable advantages compared to solid biomass. Examples are the ease of transportation, a higher energy density per volume, and lower amounts of contaminants like minerals. In addition, fast pyrolysis technology has shown to be economically viable in small scale units allowing decentralized biomass processing.
In the pyrolysis biorefinery concept, the pyrolysis oil is initially separated (primary fractionation) into two main fractions (an aqueous and an organic phase) with distinct differences in chemical composition. The aqueous phase typically contains high amounts of polar organics like sugars, organic acids, furanics, and phenolics.6,7 The organic phase mainly consists of so called pyrolytic lignin, oligomeric lignins derived from the lignin fraction in the biomass source by thermal depolymerisation. In subsequent processing steps, both separation and conversion steps are applied to provide a broad spectrum of biofuels and high added value platform chemicals and a residue that may be used for energy generation. As such, the pyrolysis oil biorefinery shows resemblances with conventional oil refineries.7 Valorisation particularly of the pyrolytic lignin fraction is still in a state of infancy though it is potentially a very interesting source for low molecular weight bio-based chemicals like aromatics and phenolics.8
Pyrolytic lignin and natural lignin differ considerably in chemical structure and molecular weight distribution. Bayerbach et al. characterized pyrolytic lignin from beech using several techniques and showed that it has an Mw of around 560–840 Da.9 This is considerably lower than for typical lignins (1600–2000 g mol−1 for Alcell hardwood lignin with THF as solvent,10 4800–5200 g mol−1 for soda bagasse lignin11). Furthermore the molecular structure of pyrolytic lignins differs considerably from natural lignins as it has already been processed thermally (450–600 °C) during the fast pyrolysis process. The main bonds in pyrolytic lignin are carbon–carbon bonds, like stilbene and diphenyl (5–5) bonds, and carbon–oxygen bonds, like phenyl coumaran (β–1) and resinol (β–β) bonds while the ether linkages typically present in natural lignin are absent12 (see Fig. 2).
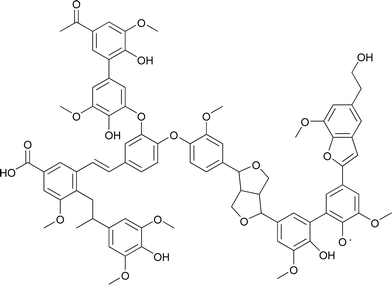 | ||
| Fig. 2 A structural model of pyrolytic lignin.12 | ||
Catalytic hydrotreatment of lignin has proven to be an interesting technology for the synthesis of (oxygenated) aromatics and a recent review was given by Zakzeski et al.13 For instance, Oasmaa et al.14 and Meier et al.15 showed that lignin may be converted to monomeric alkylphenolics like phenol, and o/p/m-cresol, albeit in relatively low yields (5–11 wt% on lignin feed). In addition, aromatics (benzene, toluene, xylene) may also be obtained.13,16
Analogous to lignin, the catalytic hydrotreatment of pyrolytic lignin may be a very interesting valorisation approach to obtain monomeric bio-based building blocks like alkylphenolics and aromatics. Compared to lignin, the use of pyrolytic lignin may have an additional advantage as the molecular weight is considerably lower, which may have a positive effect on the yields of monomeric alkylphenolics and aromatics. Very limited research has been reported on the catalytic hydrotreatment of pyrolytic lignins. Piskorz et al. explored the catalytic hydrotreatment of melted (60 °C) hog fuel pyrolytic lignin in a continuous setup with a sulfided CoMo catalyst.17 The reaction conditions were severe (415 °C and pressures of 140 bar), resulting in an oil yield of >60 wt% with an oxygen content of only 0.46%. The product oil mainly comprised cyclic/linear alkanes (62% carbon) and aromatics (38% carbon, measured by 13C-NMR). Recently Tang et al. reported the use of a supported ruthenium on SBA-15/ZrO2 catalyst for the catalytic hydrotreatment of rice husk pyrolytic lignin in the presence of a solvent (ethanol) at 260 °C, 20 bar H2 and a reaction time of 480 min.18 This resulted in oil yields up to 99 wt%, which were shown to consist mainly of phenolics, esters, and alcohols. However only relative peak areas for the various products were reported, which hampers the assessment of the yield of component classes on the lignin feed. In addition, a solvent is used, which was also shown to be reactive and contributes to the product spectrum. Mercader et al. explored the catalytic hydrotreatment of an aqueous pyrolytic lignin fraction (16.9 wt% water) from forestry residue pyrolysis oil.19 Reactions were performed at reaction temperatures of 220–310 °C and a hydrogen pressure of 190 bar, which resulted in oil yields up to 83 wt%. The chemical composition of the lignin oils was not provided.
Thus, it can be concluded that limited and scattered data are available on the catalytic hydrotreatment of pyrolytic lignin. Only one study reports the use of a solvent free system and, in addition, product yields on the lignin feed have not been reported in detail. As such, it is difficult to assess the potential of pyrolytic lignin for the synthesis of important monomeric bio-based chemicals like aromatics and alkylphenolics. Furthermore, studies comparing the performance of the catalytic hydrotreatment of pyrolytic lignin with other conventional lignin sources have not been reported to date. These studies may reveal important insights into lignin structure–product composition relations and as such will allow the sophisticated selection of the most suitable lignin sources to be used as inputs for the production of bio-based chemicals from lignin with an emphasis on aromatics and alkylphenols.
In this paper the catalytic hydrotreatment of two pyrolytic lignins from different sources (forestry residue and pine) using a supported ruthenium based catalyst in the absence of a solvent is reported. The main objective is to obtain high yields of low molecular weight compounds, like phenolics and aromatics. Ru/C was used as the catalyst. We have shown extensively already that it is a promising catalyst for the catalytic hydrotreatment of fast pyrolysis oils and lignins.20–22 Alcell lignin, an example of an organosolv lignin, was used as the benchmark to gain insights into the differences in reactivity between pyrolytic lignin and a natural lignin. Alcell lignin was selected as it has a relatively low molecular weight (≈2000 g mol−1) and a low sulphur content, which is advantageous regarding catalyst performance when using supported Ru catalysts. The experiments were carried out in a batch set-up and the resulting product oils were analysed using various techniques (GC-MS-FID, GCxGC-FID, GPC, and 13C-NMR) to gain insights into the molecular composition.
2 Material and methods
2.1 Chemicals
Ruthenium on carbon (5 wt% Ru) was obtained from Sigma Aldrich and used as received. Hydrogen (>99.99%) and nitrogen gas (>99.8%) were obtained from Hoekloos. Fast pyrolysis oils for pyrolytic lignin extractions were supplied by BTG (Netherlands, pine) and VTT (Finland, forestry residue). Alcell lignin was kindly supplied by the Wageningen University and Research Centre (WUR), the Netherlands.2.2 Separation of pyrolytic lignin from pyrolysis oil
Separation of the pyrolytic lignin from pyrolysis oil was performed by the slow addition of pyrolysis oil (40 ml, 20 ml min−1) to an excess of cold demineralized water (4 L) under intense stirring (Polytron® homogenizer) as described by Scholze et al.23 and Bayerbach.24 After the addition of the pyrolysis oil, the stirring was ceased and the pyrolytic lignin layer on top was removed and re-suspended in fresh demineralized water under gentle stirring at 200 RPM for 4 h. The suspension was filtered and the wet pyrolytic lignin was again re-suspended in fresh demineralized water and stirred gently for 12 h. The resulting pyrolytic lignin suspension was filtered on a Büchner funnel and dried under vacuum at 40 °C for 24 h. The pyrolytic lignin was ground into powder at room temperature and stored at −17 °C before use.2.3 Catalytic hydrotreatment reactions
The catalytic hydrotreatment experiments were performed in a 100 ml batch autoclave (Buchi). The autoclave has a maximum operation temperature of 400 °C and pressure of 350 bar. The reactor was surrounded by a metal block containing electrical heating elements and channels allowing the flow of cooling water. The reactor content was stirred mechanically using a Rushton type turbine with a gas induced impeller. Temperature and pressure were monitored online and logged on a PC.The reactor was filled with lignin (15 g) and catalyst (0.75 g Ru/C, 5 wt% on lignin) and subsequently flushed with hydrogen and pressurized to 120 bar at room temperature for leak testing. After the leak test, the pressure was reduced to 100 bar. Stirring was started (1200 rpm) and the reactor content was heated to 400 °C with a rate of about 8 °C min−1. The reaction time was set at zero when the pre-determined temperature was reached. The reactions were performed in a batch mode at decreasing pressure. The pressure and temperature profiles were recorded during the reactions. After the pre-determined reaction time, the reactor was cooled to room temperature with a rate of about 40 °C min−1. The pressure at room temperature was recorded for determination of the amount of gas phase components produced during reaction. Subsequently, the pressure was released to atmospheric pressure and the gas was collected in a 3 L tedlar gas bag for determination of the composition. The liquid product was collected with a syringe and weighed. The liquid phase consists of two layers: an organic layer and an aqueous layer. The aqueous and organic layers were separated by decantation. The water content of both phases was analysed using Karl Fischer titration. The reactor was rinsed several times with acetone and the combined acetone fractions were filtered and collected in a beaker. After evaporation of acetone overnight at room temperature, the weight of the remaining liquid was measured and added to the organic product.
2.4 Liquid phase analyses
GC-MS-FID analyses were performed on organic samples using a Quadruple Hewlett Packard 6890 MSD attached to a Hewlett Packard 5890 GC equipped with a 60 m × 0.25 mm i.d. and a 0.25 μm sol–gel capillary column. The injector temperature was set at 250 °C. The oven temperature was kept at 40 °C for 5 minutes then heated up to 250 °C at a rate of 3 °C min−1 and then held at 250 °C for 10 minutes.GCxGC-FID analysis was performed on organic samples with a trace GCxGC from Interscience equipped with a cryogenic trap system and two columns: a 30 m × 0.25 mm i.d. and a 0.25 μm film of RTX-1701 capillary column connected by a meltfit to a 120 cm × 0.15 mm i.d. and a 0.15 μm film Rxi-5Sil MS column. An FID detector was used. A dual jet modulator was applied using carbon dioxide to trap the samples. Helium was used as the carrier gas (continuous flow 0.6 ml min−1). The injector temperature and FID temperature were set at 250 °C. The oven temperature was kept at 40 °C for 5 minutes then heated up to 250 °C at a rate of 3 °C min−1. The pressure was set at 70 kPa at 40 °C. The modulation time was 6 seconds.
Before GCxGC-FID and GC-MS-FID analyses, the organic samples were diluted with tetrahydrofuran (THF) and 1000 ppm di-n-butyl ether (DBE) was added as an internal standard.
GPC analyses were performed on organic samples and lignin feeds using a HP1100 equipped with three 300 × 7.5 mm PLgel 3 μm MIXED-E columns in series using a GBC LC 1240 RI detector. Average molecular weight calculations were performed using the PSS WinGPC Unity software from Polymer Standards Service. The following conditions were used: THF as the eluent at a flow rate of 1 ml min−1; 140 bar, a column temperature of 42 °C, 20 μl injection volume and a 10 mg ml−1 sample concentration. Toluene was used as a flow marker.
Elemental analyses (C, H, and N) were performed using a Euro Vector 3400 CHN-S analyser. The oxygen content was determined by difference. All experiments were carried out in duplicate and the average value is provided.
The water content in the organic and aqueous samples was determined by Karl Fischer titration using a Metrohm Titrino 758 titration device. A small amount of the sample (ca. 0.03–0.05 g) was added into an isolated glass chamber containing Hydranal® (Karl Fischer Solvent, Riedel de Haen). The titrations were carried out using the Karl Fischer titrant Composit 5K (Riedel de Haen). All measurements were performed in duplicate and the average value is reported.
13C-NMR measurements were performed using a Varian Unity Plus (500 MHz) with DMSO-d6 as the solvent. At least 5000 repetitions were recorded and a relaxation delay of 2 s was applied.
2.5 Gas phase analyses
The gas phases were collected after reaction and stored in a gas bag (SKC Tedlar 3 L sample bag (9.5" × 10")) with a polypropylene septum fitting. GC-TCD analyses were performed using a Hewlett Packard 5890 Series II GC equipped with a Porablot Q Al2O3/Na2SO4 column and a molecular sieve (5 A) column. The injector temperature was set at 150 °C and the detector temperature at 90 °C. The oven temperature was kept at 40 °C for 2 minutes then heated up to 90 °C at 20 °C min−1 and kept at this temperature for 2 minutes.A reference gas was used to quantify the results (55.19% H2, 19.70% CH4, 3.00% CO, 18.10% CO2, 0.51% ethylene, 1.49% ethane, 0.51% propylene and 1.50% propane). The reference gas was used to identify the peaks by retention time and to quantify the amounts.
3 Results and discussion
3.1 Characterization of the pyrolytic lignins
Pine and forestry pyrolytic lignins (PLs) were obtained by the slow addition of representative fast pyrolysis oils of both feeds to a large excess of water. After washing and isolation, the pyrolytic lignins were obtained as brown powders, dark brown for the forest residue and light brown for pine wood. Elemental composition and molecular weight distributions (by GPC) were determined and compared to the benchmark Alcell lignin and a representative PL reported in the literature (Table 1).| Property | Alcell lignin | Pine PL | FRa PL | Beech PLb |
|---|---|---|---|---|
| a Forestry residue. b Data from Bayerbach et al.9,12 | ||||
| M w (g mol−1) | 1720 | 730 | 705 | 800 |
| Elemental composition, dry base (wt%) | ||||
| Carbon | 65.2 | 67.1 | 63.2 | 67.5 |
| Hydrogen | 5.8 | 6.8 | 6.5 | 6.0 |
| Oxygen | 29.0 | 26.1 | 30.3 | 26.3 |
| H/C ratio (mol mol−1) | 1.07 | 1.21 | 1.24 | 1.07 |
| O/C ratio (mol mol−1) | 0.33 | 0.29 | 0.36 | 0.29 |
The pyrolytic lignins used in this study are characterized by a relatively low molecular weight of around 700 g mol−1 (GPC), which is consistent with the molecular weight data reported by Bayerbach et al. for PL from beech wood.9,12 The elemental composition of the two PLs differs considerably. The carbon content of forestry residue PL is about 4 wt% lower and the oxygen content about 4 wt% higher than for the pine PL and the beech PL reported in the literature. This is expected to have an effect on the subsequent catalytic hydrotreatment reaction regarding product yields and composition.
3.2 Catalytic hydrotreatment experiments
Catalytic hydrotreatment experiment using the pyrolytic lignins and Alcell lignin were performed in a batch set-up at 400 °C, 100 bar H2 initial pressure, 4 h reaction time, using 5 wt% Ru/C as the catalyst. These harsh conditions were selected as earlier studies from our group showed that these were necessary to achieve considerable deoxygenation activity for the catalytic hydrotreatment of fast pyrolysis oils and lignins. To reduce complexity, no solvents were used and as such reactions were carried out in molten lignin.After reaction, the liquid phase consisted of two immiscible layers, a dark brown organic phase, designated as pyrolytic lignin oil (PLO), and a clear aqueous phase. For the pyrolytic lignin feeds, the organic phase stayed on top of the aqueous phase, while for the Alcell lignin the reverse was observed. The organic phases were obtained in yields up to 75 wt% on lignin feeds (Table 2). The oxygen content (6.1–7.7 wt%, Table 2) of the lignin oils is considerably lower than for the starting lignin materials (Table 1) with the largest oxygen reduction for forestry residue PL. The organic phases contain large amounts of carbon (>82.5 wt% on dry basis) and only a limited amount of water (1.4–5.8 wt%). Apparently, the organic phases contain large amounts of relatively apolar organics, which was confirmed by chromatographic and other techniques (vide infra).
| Lignin type | Pine PL | FRb PL | Alcell lignin |
|---|---|---|---|
| a 400 °C, 100 bar H2 initial pressure, 4 h, with 5 wt% Ru/C catalyst. b Forestry residue. c Excluding the carbon content of the aqueous phase. | |||
| Organic phase (wt% on lignin intake) | 75.8 | 75.4 | 64.7 |
| Aqueous phase (wt% on lignin intake) | 8.0 | 12.2 | 9.5 |
| Gas phase (wt% on lignin intake) | 7.4 | 10.9 | 11.5 |
| Carbon dioxide (mol%) | 9.1 | 15.8 | 14.5 |
| Carbon monoxide (mol%) | 3.0 | 0.6 | 2.2 |
| Ethylene (mol%) | 0.0 | 0.0 | 0.0 |
| Ethane (mol%) | 1.9 | 0.4 | 2.1 |
| Propylene (mol%) | 0.0 | 0.0 | 0.0 |
| Propane (mol%) | 1.3 | 1.9 | 0.9 |
| Methane (mol%) | 23.1 | 27.2 | 23.1 |
| Hydrogen (mol%) | 61.6 | 54.2 | 57.2 |
| Total balance (wt% on lignin intake) | 91.2 | 98.5 | 85.7 |
| Total carbon balance (%) | 97.8c | 107c | 90.4 |
| Hydrogen uptake Nl/kg lignin | 326 | 363 | 344 |
| Water content organic phase (wt%) | 2.0 | 1.4 | 5.8 |
| Carbon content aqueous phase (wt%) | n.d. | n.d. | 1.5 |
| Elemental composition lignin oil (wt%, dry basis) | |||
| Carbon | 82.9 | 84.5 | 82.5 |
| Hydrogen | 9.5 | 9.4 | 9.5 |
| Oxygen | 6.4 | 6.1 | 7.7 |
The amount of the aqueous phases after reaction is between 8 and 12.2 wt% on lignin intake and they contain only limited amounts of carbon (1.5 wt% for Alcell). The formation of water is indicative of the occurrence of the desired hydrodeoxygenation reaction. Only minor amounts of solid residues were observed after reaction. These amounts were that limited that quantification by separation from the catalysts after reaction proved impossible.
Good mass balance closure was observed for most experiments, above 92 wt% for the total mass balances for pyrolytic lignins and >90% for the carbon balances.
4 Composition of the gas and liquid phases
4.1 Gas phase composition
The gas phase composition after reaction for all three lignin sources was about similar and contained, besides hydrogen, mainly CH4 (>23 mol%) and CO2 (>9.1 mol%) (see Table 2 for details). The presence of significant amounts of hydrogen after reaction (>54 mol%) indicate that the reactions were not carried out under hydrogen starvation conditions.A possible pathway for methane formation is the occurrence of gas phase reactions between CO/CO2 and hydrogen (eqn (1)). These reactions are known to be catalysed by supported Ru catalysts.20,25,26
| CO + 3H2 ↔ CH4 + H2O, CO2 + 4H2 ↔ CH4 + 2H2O | (1) |
The required CO2 and CO are likely formed by gasification reactions of various reactive lignin fragments. For instance, it is well known that Ru catalysts are active for the gasification of Alcell lignin in supercritical water at 400 °C (3.3 wt% Alcell in water).27 Though in our case the water content is much lower, such gasification reactions may occur to a significant extent.
Another pathway for methane formation is the hydrogenolysis of methoxy groups to methane and a substituted phenol (eqn (2))
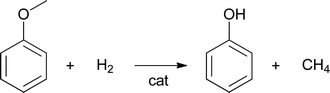 | (2) |
Analyses of the pyrolytic feeds by NMR (vide infra) reveal that methoxy groups are indeed present in the pyrolytic lignin feeds to a significant extent. Hydrogenolysis of methoxy groups has been shown to occur for model reactions of anisole and guaiacol with hydrogen using a NiMo on SiO2–Al2O3 catalyst at 250–350 °C.28 Model reactions with guaiacol in water at 150–250 °C using Ru/C showed primarily the formation of 2-methoxycyclohexanol, cyclohexanediol, and cyclohexanol (Scheme 1).29 Thus besides methoxy removal by hydrogenolysis, Ru/C also shows a strong tendency to hydrogenate the aromatic carbon–carbon double bonds.
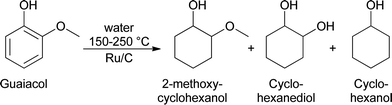 | ||
| Scheme 1 The reaction pathway for the catalytic hydrotreatment of guaiacol with Ru/C.29 | ||
However, temperatures in the model component study using Ru/C are lower than in this study (400 °C), making comparison cumbersome.
4.2 Composition of the pyrolytic lignin oils after catalytic hydrotreatment
The elemental composition of feeds and the lignin oils were determined (Tables 1 and 2) and the results are shown in Fig. 3 in the form of a van Krevelen plot. In addition, literature data on the elemental composition of pyrolytic lignins are also provided. Clearly, the elemental composition of pyrolytic lignins covers a wide range of H/C and O/C ratios (0.2–0.4 for O/C and 1.0–1.3 for H/C). The forestry residue PL used in this study has a relatively high O/C ratio (0.36), while that of pine PL is considerably lower (0.29). Thus, the elemental composition of pyrolytic lignins in general differs significantly and this is likely due to differences in extraction methods and the type of pyrolysis oil used.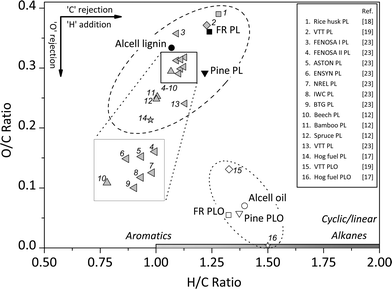 | ||
| Fig. 3 The van Krevelen plot for the feeds and products of the catalytic hydrotreatment reaction (400 °C, 100 bar H2 initial pressure, 4 h, 5 wt% of Ru/C) and literature data for PL. | ||
After the catalytic hydrotreatment reactions of the two pyrolytic lignins used in this study, the O/C ratio is reduced considerably to values of about 0.07. The H/C ratio of the products is between 1.3 and 1.4 and slightly higher than in the feeds (Fig. 3). Thus, hydrodeoxygenation reactions have occurred to a significant extent. In Fig. 3, the elemental compositions of two hydrotreated pyrolytic lignins reported in the literature are also provided (entries 15 and 16). These differ considerably from our data. The much higher oxygen content for entry 15 is likely due to less severe reaction conditions (220–310 °C) and the high water content in the starting pyrolytic lignin (16.9 wt%).19 Entry 16, an oil from the catalytic hydrotreatment of hog fuel obtained in a continuous setup with sulphided CoMo as the catalyst under severe reaction conditions (415 °C and pressures of 140 bar), has a very low oxygen content and a higher H/C ratio.17 It is well known that sulphided CoMo is a better catalyst for the deep hydrotreatment than Ru/C, particularly when used in a continuous set-up with low weight hourly space velocities.
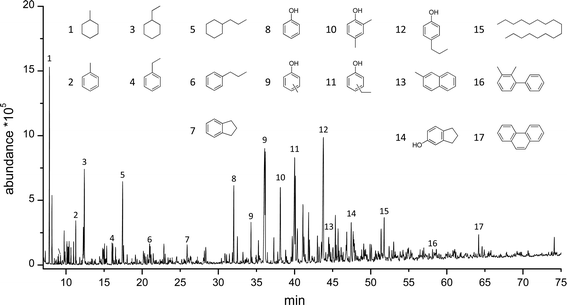
The pyrolytic lignin oils and the Alcell lignin oil after hydrotreatment were analysed using GCxGC-FID and GC-MS-FID to determine the product composition. A representative example of a GC-MS-FID chromatogram is given in Fig. 4, showing a large number of volatile components in the mixture (>400). Clearly, large amounts of (alkyl substituted) phenolics, aromatics and alkanes (cyclic and linear) are present.
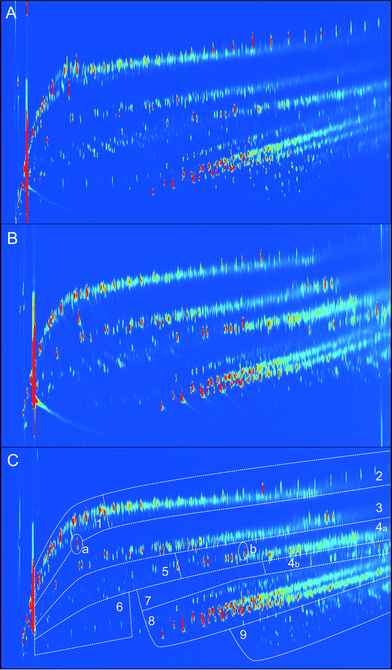
To gain more insights into the various product classes present in the oil, GCxGC-FID was applied. It allows rapid assessment of major organic compound classes in an organic biomass derived sample.30,31 Representative chromatograms of the products are given in Fig. 5, including the selected product classes. Clearly, good separation between the various compound classes is possible and discrete regions are visible.
Quantification of the various product classes was performed and the results are given in Table 3. The main GCxGC detectable products in the different lignin oils are alkylphenolics, followed by aromatics, linear/branched alkanes, and cyclic alkanes.
| Compound classes | Composition of product oils | ||
|---|---|---|---|
| Pine | FRb | Alcell | |
| a Hydrotreatment conditions: 400 °C, 100 bar H2 initial intake, 4 h, with 5 wt% Ru/C. b Forestry residue. c wt% on lignin feed intake. | |||
| Alkylphenolicsc | 18.9 | 20.5 | 6.6 |
| Guaiacols | 0.3 | 0.1 | 2.7 |
| Catechols | 0.1 | 0.6 | 0.5 |
| Aromatics | 7.3 | 14.1 | 9.7 |
| Linear/branched alkanes | 5.1 | 7.5 | 5.6 |
| Cyclic ring alkanes | 7.3 | 6.7 | 3.7 |
| Ketones/alcohols | 0.8 | 1.9 | 1.7 |
| Total GCxGC | 39.8 | 51.3 | 30.6 |
The largest amount of total GC detectable components was found in the forestry residue PLO (51.3 wt% on lignin feed intake), whereas the amount in Alcell lignin oil was considerably lower (30.6 wt%). A likely explanation is the molecular weight difference between the PL lignin and Alcell lignin, with the lower molecular weights for pyrolytic lignins leading to higher amounts of lower molecular weight, volatile and thus GC detectable products (vide infra). Remarkably, the amounts of alkylphenolics are much higher in the pyrolytic lignin oils than in the Alcell lignin oil. Actually, the amount of alkylphenolics in FR lignin oil is a factor of three higher than in the Alcell lignin oil. Thus, it may be concluded that pyrolytic lignins are by far better sources for low molecular weight products and particularly alkylphenolics than Alcell lignin.
The main individual components in the various product classes (alkylphenolics, aromatics, aliphatics) in forestry residue PLO are given in Table 4.
| Compounds | Amounta | Structure |
|---|---|---|
| a wt% on lignin intake. | ||
| Alkylphenolics |
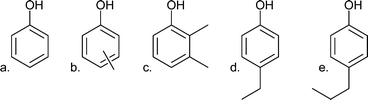
|
|
| a. Phenol | 0.58 | |
| b. m/p-Cresol | 2.46 | |
| c. 2,3-Dimethylphenol | 0.85 | |
| d. 4-Ethylphenol | 2.07 | |
| e. 4-Propylphenol | 2.02 | |
| Aromatics |
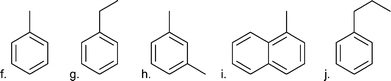
|
|
| f. Toluene | 0.47 | |
| g. Ethylbenzene | 0.21 | |
| h. m-Xylene | 0.13 | |
| i. Methylnapthalene | 0.44 | |
| j. Propylbenzene | 0.16 | |
| Cyclic/linear/branched alkanes |
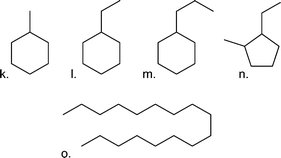
|
|
| k. Methylcyclohexane | 1.11 | |
| l. Ethylcyclohexane | 0.93 | |
| m. Propylcyclohexane | 0.79 | |
| n. 1-Ethyl-2-methylcyclopentane | 0.70 | |
| o. Heptadecane | 0.63 | |
The total amount of detectable species using the GCxGC technique is at max. 51 wt%, indicating the presence of large amounts of non-GC detectable compounds, presumably of higher molecular weight. This was confirmed by performing GPC analyses on the product oils and the starting lignin feeds (see Fig. 6 for details).
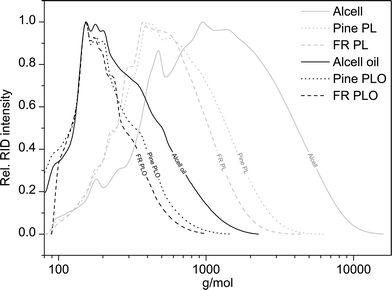 | ||
| Fig. 6 Molecular weight distribution of pine PL, forestry residue PL, organosolv Alcell lignin, and the corresponding hydrotreated lignin oils (400 °C, 4 h, 100 bar initial H2, 5 wt% Ru/C). | ||
Clearly, the molecular weight of the lignin oils is significantly lower than the lignin feeds, indicating that all three lignin feeds are significantly depolymerised during the catalytic hydrotreatment reactions. The molecular weights of the pyrolytic lignin oils are remarkably lower than found for Alcell lignin oil, which is attributed to the already lower molecular weight of the pyrolytic lignins, and likely also to differences in the type of linkages between the aromatic rings in the pyrolytic lignins and Alcell lignin. Furthermore, a clear correlation is present between the total GCxGC detectable and the molecular weight of the product oils, with higher molecular weights leading to a lower percentage of GC detectable product oils (figure not shown).
The molecular composition of the lignin oils was also determined by 13C-NMR. Due to the complex nature of the starting feeds and the resulting lignin oils, semi-quantification was performed by dividing the 13C-NMR in characteristic regions based on methods from Hu et al.,32 Ingram et al.,33 Xia et al.,34 and Robert.35 The normalized integration results for both the starting materials and the resulting lignin oils are given in Table 5. The 13C-NMR spectra of Alcell lignin and pine PL and their hydrotreated product oils are shown in Fig. 7.
| Chemical shift region (ppm) | Type of carbon | Carbon content (% of total carbon) | |||||
|---|---|---|---|---|---|---|---|
| Alcell | FRa | Pine | |||||
| Lignin | LO | PL | PLO | PL | PLO | ||
| a Forestry residue. b The ratio of the area of aliphatic carbons (δ 36–0 ppm) and aromatic carbons (δ 160–100 ppm). | |||||||
| 0–36 | Aliphatic chains | 17.1 | 47.0 | 23.0 | 44.3 | 21.7 | 45.3 |
| 52–58 | Methoxy groups | 16.6 | 0.7 | 8.1 | 0.0 | 9.1 | 0.1 |
| 58–100 | Ether bonds (α, β and γ) | 6.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 100–160 | Total aromatics | 59.5 | 51.9 | 68.8 | 55.8 | 69.3 | 54.6 |
| 100–123 | Non branched aromatics | 25.1 | 17.9 | 27.5 | 19.7 | 32.8 | 23.0 |
| 123–160 | Branched aromatics | 34.4 | 34.0 | 41.3 | 36.1 | 36.5 | 31.6 |
| 160–195 | Carbonyl structures | 0.5 | 0.3 | 0.1 | 0.0 | 0.0 | 0.0 |
| Aliphatic/aromatic ratiob | 0.29 | 0.91 | 0.33 | 0.79 | 0.31 | 0.83 | |
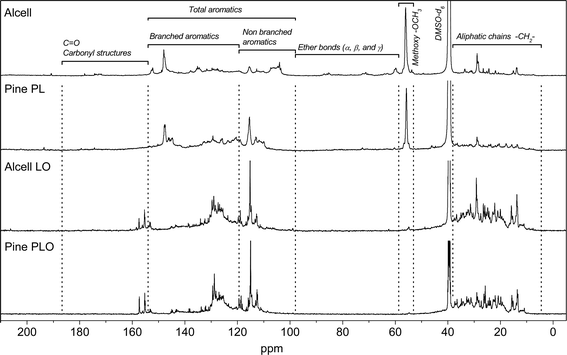 | ||
| Fig. 7 13C-NMR spectra of two feeds (Alcell and PL from pine) and their product oils after catalytic hydrotreatment. | ||
The 13C-NMR spectra reveal that the pyrolytic lignins and Alcell lignin have different molecular compositions (Fig. 7, Table 5). For instance, clear resonances of ether bonds (α, β, γ) between the aromatic rings (δ 58–100 ppm range) are absent in pyrolytic lignins, whereas they are present in Alcell lignin in considerable amounts. This has also been reported in the literature and is contributed to the harsh conditions during the fast pyrolysis process where the ether bond linkages in lignin are easily cleaved and other linkages are formed (e.g. diphenyl, and stilbene type carbon–carbon bonds)12 upon repolymerisation of reactive fragments in the vapour phase.
The 13C-NMR data for the product oils after catalytic hydrotreatment are given in Fig. 7 and Table 5. The products from the pyrolytic lignins show an almost complete removal of the methoxy groups, while the amount in the product oil from the Alcell lignin feed is considerably higher. This is in agreement with the product composition results from GCxGC analyses. Here limited amounts of methoxy compounds were observed in the PLO (0.1–0.3 wt%) whereas the amount in Alcell lignin oil was considerably higher (2.7 wt%) (see Table 3 for details). For all product oils, the aliphatic-to-aromatic ratio, defined as the ratio of the area of aliphatic carbons (δ 36–0 ppm) and aromatic carbons (δ 160–100 ppm), is higher than the corresponding feeds, indicative of substantial hydro(deoxy)genation activity of the catalyst. This is supported by elemental analyses and GC data (vide infra).
5 Discussion
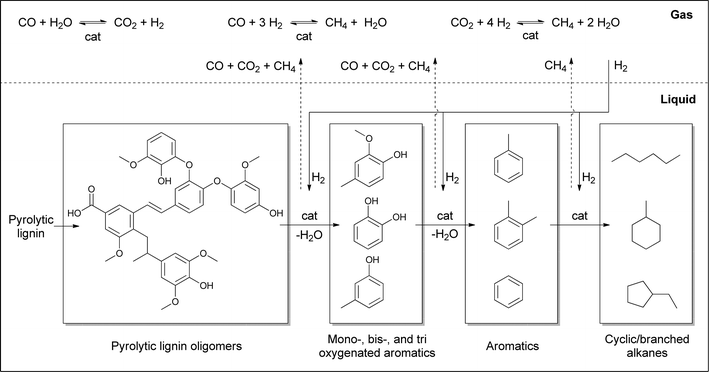
Based on the chemical composition (GC, NMR), the molecular weight distributions of the product lignin oils (GPC), and the gas phase composition, a global reaction network may be established for the catalytic hydrotreatment of pyrolytic lignins (Fig. 8). The initial step involves the thermal and/or catalytic breakdown of the pyrolytic lignin feeds to smaller molecular weight fragments. Initially, likely the more labile C–O–C linkages are broken, while in a later stage, also considerable C–C bond cleavage occurs. The latter reactions are important as the extent of C–O–C linkages in pyrolytic lignin is limited. Repolymerisation of reactive fragments ultimately leading to char, as observed frequently during the catalytic hydrotreatment of lignin, does not occur to a significant extent as char formation was very limited. This is not surprising as the lignins in the pyrolytic lignin feed have already undergone a thermal treatment during the fast pyrolysis process, where the most reactive fragments have already been converted into char and do not end up in the fast pyrolysis oil.The oligomers are depolymerised to oxygenated aromatics which are subsequently converted by catalytic hydrodeoxygenation reactions to aromatics and/or alkanes. Alkane formation may either occur directly from the oxygenated aromatics by hydrogenation of aromatic C–C double bonds followed by dehydration reactions or by cleavage of O–C bonds or a combination of the two. The exact extent of these reactions is not possible to assess without detailed product composition–time studies.
The desired products of this study are low molecular weight alkylphenolics and aromatics, which are important industrial chemicals and have higher economic values (>1000 euro per ton) than aliphatic fuel components (<600 euro per ton). As such, hydrogenation of the aromatic rings should be avoided and apparently, the Ru/C catalyst used in this study is too active for this purpose under the conditions employed. Therefore, further catalyst screening studies and process optimization studies will be required to minimise C–C double bond hydrogenation and as such reduce alkane formation.
Of interest is the considerably higher amounts of monomeric aromatics and alkylphenolics in the product oils when using pyrolytic lignin as the feed instead of Alcell lignin. This is likely caused by the lower molecular weights of the pyrolytic lignins. As such, fast pyrolysis technology may be considered as an attractive depolymerisation technique for lignins, similar to conventional base catalysed depolymerisation processes. Another striking feature is the by far higher amounts of alkylphenolics in the product oils when using pyrolytic lignins as feeds instead of Alcell lignin. This may be molecular weight related but also may be due to the different types of linkages between the aromatic rings in pyrolytic and Alcell lignins. The latter is expected to have a considerable effect on the rate of formation of monomeric fragments. Further studies with an extended range of (pyrolytic) lignin feeds are required to establish clear lignin structure–product composition relations.
6 Conclusions
The catalytic hydrotreatment of two representative pyrolytic lignins (forestry residue and pine) to low molecular weight aromatics and alkylphenolics was investigated and the results were compared with Alcell lignin. The best results were obtained with a forestry residue PL feed which resulted in high product oil yields (>75 wt%), containing high yields of valuable chemicals like alkylphenolics (20.5 wt% on lignin feed) and aromatics (14.1 wt% on lignin feed). Remarkably, and the most relevant finding of this paper is that the amount of alkylphenolics in the product oil of forestry residue PL is three times higher than for Alcell lignin under similar conditions. Thus, the PL fraction of phase separated fast pyrolysis oils is an attractive feed for the synthesis of important chemical building blocks like alkylphenolics and aromatics. The mixture of alkylphenolics may be used as a phenol replacement in various phenol based resins (e.g. phenol-formaldehyde). As such, these findings may have a positive effect on the techno-economic potential of pyrolysis oil biorefineries and as such be a stimulus for further developments in this field.Acknowledgements
Hans van der Velde (University of Groningen, Stratingh Institute) is acknowledged for performing the elemental analyses and Wim Kruizinga for help with the 13C-NMR measurements. This work was conducted in the framework of the Dutch national project “LIGNOVALUE”, contract no. EOSLT-05011. Financial support from AgentschapNL is gratefully acknowledged.References
- M. Balat, H. Balat and C. Oz, Prog. Energy Combust. Sci., 2008, 34, 551–573 CrossRef CAS PubMed.
- A. E. Atabani, A. S. Silitonga, I. A. Badruddin, T. M. I. Mahlia, H. H. Masjuki and S. Mekhilef, Renewable Sustainable Energy Rev., 2012, 16, 2070–2093 CrossRef PubMed.
- J. J. Bozell, Clean: Soil, Air, Water, 2008, 36, 641–647 CrossRef CAS.
- T. Dickerson and J. Soria, Energies, 2013, 6, 514–538 CrossRef CAS PubMed.
- F. Cherubini, Energy Convers. Manage., 2010, 51, 1412–1421 CrossRef CAS PubMed.
- C. R. Vitasari, G. W. Meindersma and A. B. de Haan, Bioresour. Technol., 2011, 102, 7204–7210 CrossRef CAS PubMed.
- R. H. Venderbosch and W. Prins, Biofuels, Bioprod. Biorefin., 2010, 4, 178–208 CrossRef CAS.
- J. E. Holladay, J. F. White, J. J. Bozel and D. Johnson, Top Value-Added Chemicals From Biomass: Volume II-Results of Screening for Potential Candidates from Biorefinery Lignin, 2007, Technical Report PNNL-16983 Search PubMed.
- R. Bayerbach, V. D. Nguyen, U. Schurr and D. Meier, J. Anal. Appl. Pyrolysis, 2006, 77, 95–101 CrossRef CAS PubMed.
- H. L. Hergert, G. C. Goyal and J. H. Lora, in Lignin: Historical, Biological, and Materials Perspectives, ed. W. G. Glasser, R. A. Northey and T. P. Schultz, American Chemical Society, 1999, ch. 12, vol. 742, pp. 265–277 Search PubMed.
- S. Baumberger, A. Abaecherli, M. Fasching, G. Gellerstedt, R. Gosselink, B. Hortling, J. Li, B. Saake and E. de Jong, Holzforschung, 2007, 61, 459–468 CrossRef CAS.
- R. Bayerbach and D. Meier, J. Anal. Appl. Pyrolysis, 2009, 85, 98–107 CrossRef CAS PubMed.
- J. Zakzeski, P. C. A. Bruijnincx, A. L. Jongerius and B. M. Weckhuysen, Chem. Rev., 2010, 110, 3552–3599 CrossRef CAS PubMed.
- A. Oasmaa, R. Alen and D. Meier, Bioresour. Technol., 1993, 45, 189–194 CrossRef CAS.
- D. Meier, R. Ante and O. Faix, Bioresour. Technol., 1992, 40, 171–177 CrossRef CAS.
- C. Amen-Chen, H. Pakdel and C. Roy, Bioresour. Technol., 2001, 79, 277–299 CrossRef CAS.
- J. Piskorz, P. Majerski, D. Radlein and D. S. Scott, Energy Fuels, 1989, 3, 723–726 CrossRef CAS.
- Z. Tang, Y. Zhang and Q. Guo, Ind. Eng. Chem. Res., 2010, 49, 2040–2046 CrossRef CAS.
- F. D. M. Mercader, M. J. Groeneveld, S. R. A. Kersten, C. Geantet, G. Toussaint, N. W. J. Way, C. J. Schaverien and K. J. A. Hogendoorn, Energy Environ. Sci., 2011, 4, 985–997 CAS.
- J. Wildschut, M. Iqbal, F. H. Mahfud, I. Melian-Cabrera, R. H. Venderbosch and H. J. Heeres, Energy Environ. Sci., 2010, 3, 962–970 CAS.
- J. Wildschut, I. Melian-Cabrera and H. J. Heeres, Appl. Catal., B, 2010, 99, 298–306 CrossRef CAS PubMed.
- P. de Wild, R. Van der Laan, A. Kloekhorst and E. Heeres, Environ. Prog. Sustainable Energy, 2009, 28, 461–469 CrossRef CAS.
- B. Scholze and D. Meier, J. Anal. Appl. Pyrolysis, 2001, 60, 41–54 CrossRef CAS.
- R. Bayerbach, Ph.D. Thesis, Universität Hamburg, 2006 Search PubMed.
- D. C. Elliott, L. J. Sealock and E. G. Baker, Ind. Eng. Chem. Res., 1993, 32, 1542–1548 CrossRef CAS.
- R. H. Venderbosch, A. R. Ardiyanti, J. Wildschut, A. Oasmaa and H. J. Heeresb, J. Chem. Technol. Biotechnol., 2010, 85, 674–686 CrossRef CAS.
- M. Osada, O. Sato, K. Arai and M. Shirai, Energy Fuels, 2006, 20, 2337–2343 CrossRef CAS.
- J. B. S. Bredenberg, M. Huuska, J. Raty and M. Korpio, J. Catal., 1982, 77, 242–247 CrossRef CAS.
- D. C. Elliott and T. R. Hart, Energy Fuels, 2009, 23, 631–637 CrossRef CAS.
- J. H. Marsman, A. Kloekhorst, L. Rohrbach and H. J. Heeres, J. Chromatogr. A Search PubMed submitted.
- J. H. Marsman, J. Wildschut, F. Mahfud and H. J. Heeres, J. Chromatogr. A, 2007, 1150, 21–27 CrossRef CAS PubMed.
- J. Hu, R. Xiao, D. Shen and H. Zhang, Bioresour. Technol., 2013, 128, 633–639 CrossRef CAS PubMed.
- L. Ingram, D. Mohan, M. Bricka, P. Steele, D. Strobel, D. Crocker, B. Mitchell, J. Mohammad, K. Cantrell and C. U. Pittman, Energy Fuels, 2008, 22, 614–625 CrossRef CAS.
- Z. Xia, L. Akim and D. Argyropoulos, J. Agric. Food Chem., 2001, 49, 3573–3578 CrossRef CAS PubMed.
- D. Robert, in Methods in lignin chemistry, ed. S. Lin and C. Dence, Springer Berlin Heidelberg, 1992, ch. 5, pp. 250–273 Search PubMed.
| This journal is © The Royal Society of Chemistry 2014 |