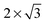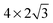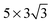Interaction of H2O and H2S with Cu(111) and the impact of the electric field: the rotating & translating adsorbate, and the rippled surface
Jin Hyun
Chang
*a,
Ahmed
Huzayyin
ab,
Keryn
Lian
c and
Francis
Dawson
a
aThe Edward S. Rogers Sr. Department of Electrical & Computer Engineering, University of Toronto, 10 Kings College Road, Toronto, Canada. E-mail: jin.chang@mail.utoronto.ca
bElectrical Power & Machines Department, Cairo University, Giza, Egypt
cDepartment of Materials Science & Engineering, University of Toronto, 184 College Street, Toronto, Canada
First published on 3rd November 2014
Abstract
The interactions of H2O and H2S monomers with Cu(111) in the absence and presence of an external electric field are studied using density functional theory. It is found that the adsorption is accompanied by a rippled pattern of the surface Cu atoms and electron accumulation on the surface Cu atoms surrounding the adsorption site. The response of the H2O/Cu(111) and H2S/Cu(111) interfaces to the external electric field is computed up to the field magnitude of 1010 V m−1. The results show that H2O rotates and translates much more with an electric field than H2S does. The extent of the surface deformation changes considerably with the applied electric field, which influences the translation pattern of the adsorbates. On the other hand, the rotation of the adsorbates is correlated to the dipole moment of the molecules and their adsorption energies.
1 Introduction
Understanding the nature of the interaction between the electrode and adsorbates is of great importance in many fields of applied science and engineering. Several relevant applications include corrosion prevention, hydrogen production and electrocatalytic energy conversion. However, understanding the physics of the interface on an atomic or electronic scale remains a challenging task.1–3 One of the primary sources of the challenge is the presence of a high electric field at the interface. The electric field magnitudes reach in the order of 1010 V m−1 when a voltage is applied or current is injected into the electrode.1The interaction between monomers and the electrode in the presence of an external electric field is directly linked to many technological processes involving electrocatalytic reactions.4–7 The monomeric adsorption of water-like molecules is difficult to characterize experimentally due to their tendency to form clusters.2,8 Consequently, the characterization of monomer–electrode interactions that specifically includes the impact of the electric field relies on computational studies.1 However, a comprehensive understanding of the behaviour of the interface in the presence of an external field is a major goal yet to be achieved.3
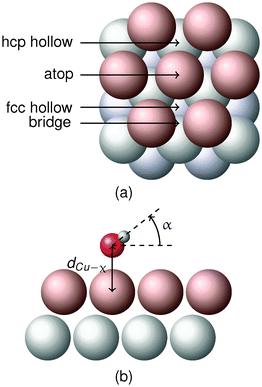
We have recently investigated the nature of the interaction between a water monomer and gold electrodes, Au(111) and Au(110), with and without an external electric field using density functional theory (DFT).9 The Au–H2O interaction was explained in terms of the interplay between covalent binding and electrostatic interactions. The covalent binding involves the interaction between the Au atoms, mainly their d orbital, with a 1b1 orbital (the lone pair orbitals of the O atom) and the 3a1 orbital (attributed to orbitals of both O and H atoms) of the water monomer. These were, respectively, referred to as Au–O and Au–OH interactions. The electrostatic interaction is due to the interaction of the water dipole with the surface and the imposed electric field. The correlation between the adsorption angle and the contribution of Au–O and Au–OH interactions was also determined, where the adsorption angle α represents a vertical angle between the plane parallel to the electrode surface and a vector connecting O and H as shown in Fig. 1. The Au–O interaction was found to play a greater role in the overall water–electrode interaction than the Au–OH interaction for the molecule in a flat orientation (|α| is small) compared to the molecule in a vertical orientation (|α| is large) and vice versa.
The analysis of the adsorption of the water monomer onto Au(111) and Au(110)9 further showed that the covalent interaction between the water monomer and the gold surface is the dominant type of interaction in the absence of an electric field whereas the electrostatic interaction dominates at higher electric fields. When the electrostatic interaction becomes comparable to the covalent interaction, the H2O molecule begins to rotate and its dipole orientation asymptotically approaches an alignment oriented in the direction of the electric field.
The present work aims at extending the understanding of the adsorbate–electrode interaction by investigating different electrode and adsorbate pairs using DFT. A copper electrode with (111) surface, Cu(111), was selected for the electrode because it has a similar electronic structure as that of gold but displays significantly different interfacial characteristics such as chemical reactivity and mechanical rigidity. In particular, the gold electrodes remained structurally insensitive to adsorption and the applied electric field.9 The lack of a large structural change in the gold surfaces aligns well with the comparison made by Michaelides10 where gold was one of the least structurally sensitive metals to water adsorption among the following metals: Ru, Rh, Pd, Pt, Cu, Ag and Au. Copper, on the other hand, was the most structurally sensitive metal to the adsorption of H2O.10 By analyzing the monomeric adsorption onto Cu(111), one can assess not only the applicability of the aforementioned characteristics of H2O/Au, but also the contribution of the surface structure change to the overall behaviour of the adsorbate/electrode interface.
On the adsorbate side, hydrogen sulfide was investigated in addition to the water monomer because it has the same number of lone pairs as water, is similar in size and structure, and has a different dipole moment compared to water as shown in Table 1. Particularly, the lone-pair orbital is known to contribute the most to the overall adsorbate–electrode interaction for both water and hydrogen sulfide8,11 and the number of lone pairs of the two are the same. On the other hand, the dipole moments of the two molecules differ significantly (1.850 D for H2O and 0.970 D for H2S). The dipole moment is expected to play an increasing role when an electric field is applied. The dipole–field interaction is the greatest contributor to the electrostatic interaction in the presence of the electric field and it increases as the magnitude of the electric field increases.9
A prime application of H2O/Cu(111) is hydrogen production using a water gas shift reaction where a copper-based catalyst is widely used.15,16 On the other hand, understanding the interaction between H2S and copper is important in making copper-based catalysts – H2S is a common impurity which promotes the formation of a S overlayer.11,17 The tarnishing of copper also involves the H2S/Cu interaction associated with the copper sulfidation process.18 There are many other technological applications that can benefit from gaining further insights into the interaction between H2O/H2S and copper.
The magnitude of the electric field is increased in 109 V m−1 increments, in both positive and negative directions, with a maximum electric field magnitude of 1010 V m−1. The responses of the adsorbates to the external field, namely rotation, translation and structural changes, are compared and linked to the nature of the adsorption without an external field. We demonstrate that the response of the adsorbate is nonlinear and asymmetric with respect to the magnitude and direction of the electric field and is influenced by the structural sensitivity of the electrode surface to the adsorption. The rotation of the adsorbates can be predicted from the dipole moment of the molecule and its adsorption energy. The adsorption of H2O and H2S is accompanied by a rippled pattern of the surface Cu atoms and the extent of the surface deformation needs to be considered to describe the translation pattern of the adsorbates. The rippled pattern of the surface atoms and its impact on the response of the interface to the external electric field have not been observed previously and are reported for the first time.
2 Computational methods
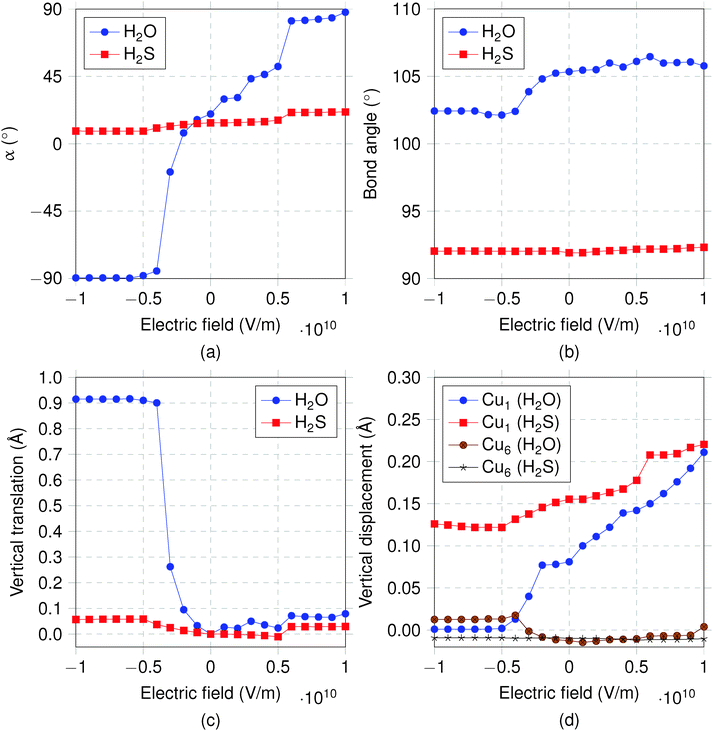
The results presented in this work are based on DFT computations using the Quantum ESPRESSO package,19 which uses plane wave basis sets and applies periodic boundary conditions. The Perdew–Burke–Ernzerhof20 exchange–correlation functional was used with the projector augmented wave type21 pseudopotentials as constructed by Kresse and Joubert.22 The cutoff energy for wave functions and charge density were 70 Ry and 560 Ry, respectively. The surface was modelled using a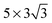 supercell, which corresponds to a 1/30 monolayer surface coverage. This structure is larger than any of the reported DFT investigations of Cu(111) and was chosen to avoid any artificial lateral interaction with its images created by periodic boundary conditions. The next section will show that a large lateral size of the supercell is critical in characterizing the electrode–adsorbate interaction both with and without the electric field. The slabs were separated by a vacuum greater than 20 Å to avoid the vertical interactions.
supercell, which corresponds to a 1/30 monolayer surface coverage. This structure is larger than any of the reported DFT investigations of Cu(111) and was chosen to avoid any artificial lateral interaction with its images created by periodic boundary conditions. The next section will show that a large lateral size of the supercell is critical in characterizing the electrode–adsorbate interaction both with and without the electric field. The slabs were separated by a vacuum greater than 20 Å to avoid the vertical interactions.
All of the results reported were computed using a slab consisting of 5 layers of Cu where the top 3 layers were fully relaxed while the bottom 2 layers were fixed at the bulk atomic coordinates, unless explicitly stated otherwise. Computations with a higher number of layers have been compared and the comparison confirmed the convergence and validity of the settings used. The dipole created by the lack of symmetry was compensated for by the insertion of the dipole layer in the middle of the vacuum,23 which was also used to introduce an external electric field. A Monkhorst–Pack type k-point grid24 of 3 × 3 × 1 was used for sampling the supercell. All of the values above were selected to ensure that the total energy converges within 0.01 eV. The validity of the DFT modeling approach was ensured by comparing parameters such as the bond lengths and angles of H2O and H2S, and the lattice constant of Cu and its work function to experimental values as shown in Table 2. The work function, Φ, was computed using the “bulk plus band line up” method.25 The enthalpy of reaction, ΔH, corresponds to the change in the energy (or heat) accompanied by the reaction. The reactions are H2O → H2 + ½O2 for water and H2S → H2 + ½S2 for hydrogen sulfide. The enthalpy of reaction was computed as ΔH = ΔE + ΔZPE, where ΔE is the reaction energy and ΔZPE is the change in the zero-point energy (ZPE). E corresponds to the total electronic energy at the ground state and it was computed by DFT via structural optimization to yield the minimum energy on the potential energy surface. The experimental values of ΔH were obtained by subtracting the sum of enthalpies of formation of reactants from the sum of enthalpies of formation of products at 0 K. The values of enthalpies of formation and ZPE are as reported in ref. 26. Given the expected level of accuracy of DFT (structural and energetic accuracies of around 3% and 10–20%,27 respectively), the agreement between computed values and those obtained from experiment is satisfactory.
3 Results & discussion
3.1 Adsorption without an electric field
It is known that the atop site (Fig. 1a) is the most stable adsorption site for both H2O10 and H2S30 on Cu(111) and other face-centered cubic (fcc) (111) surfaces. Therefore, only an atop site is considered for adsorption for both monomers since the monomers would preferentially be adsorbed onto an atop site. The most stable adsorbed structures of the monomers are determined through structural optimization computations using various initial orientations of the monomers on the atop site. A comparison between the adsorbed structures and adsorption energies of H2O and H2S at the atop site determined in the present work and previous work by others4,10,11,17,31–33 is shown in Table 3. Eads is the adsorption energy in eV. α (Fig. 1b), the adsorption angle, represents the vertical angle between the plane parallel to the electrode surface and the vector connecting the atom “χ” (the symbol χ stands for O in the case of water and S in the case of hydrogen sulfide) and H. α is defined so that a positive angle corresponds to a “hydrogen-up” orientation while a negative angle corresponds to a “hydrogen-down” orientation. dCu–χ (Fig. 1b) represents the distance between χ and the surface Cu atom that is immediately underneath the adsorbate. The lateral size of the supercell used for the results is shown in terms of the effective surface coverage (Θ) of adsorbate.| Adsorbate | Source | E ads (eV) | α (deg) | d Cu–χ (Å) | Θ (ML) |
|---|---|---|---|---|---|
| a The authors used multiple cell sizes for comparison. The results with the largest cell size is shown here. b Originally reported in kJ mol−1 and converted to eV. | |||||
| H2O | This work | −0.19 | 19.89 | 2.32 | 1/30 |
| Ref. 31 | −0.18 | 20.8 | 2.33 | 1/20 | |
| Ref. 4 | −0.21 | 7 | 2.34 | 1/9 | |
| Ref. 32 | −0.24 | 3 | 2.40 | 1/9 | |
| Ref. 10 | −0.24 | 15 | 2.25 | 1/4 | |
| Ref. 33 | −0.19b | 8.7 | 2.36 | 1/4 | |
| H2S | This work | −0.25 | 14.00 | 2.46 | 1/30 |
| Ref. 11 | −0.23 | 13 | 2.51 | 1/9 | |
| Ref. 17 | −0.28 | 13.6 | 2.41 | 1/9 | |
It is known that the modelling parameters such as the size of the cell and the effective surface coverage influence the computation results.34,35 The lateral size of the cell is particularly important in studying the adsorption of polar molecules.35 Considering the large variations in the lateral sizes of the cells used for the results shown in Table 3, the results of this work show an acceptable agreement with other DFT computation results. It was previously mentioned that the lateral size of the cell in this work is larger than any of the reported results. The results are in much better agreement if we compare our results with that of the work employing the next largest lateral size.11,17,31 In particular, the adsorption energy and geometry of water found in this work and by Nadler and Sanz31 are almost the same. The remaining results show larger variations in both Eads and adsorption geometry due to the smaller lateral size of the cell.
It has been reported that the potential energy surface (PES) is relatively flat and as a result, small variations in water geometry (e.g., α and dCu–χ) have a little impact on Eads.36 This was also shown to be true when Nadler and Sanz37 compared the impact of cell size on the adsorption of the water monomer onto Au(111) – different cell sizes cause the geometry of water to change while Eads remains insensitive. The difference in both the geometry and adsorption energy as the size of the cell is varied indicates that the artificial interactions with the periodic images may be severe enough to distort the PES when the cell size is too small. The detailed analysis, to follow, shows that the adsorbate–electrode interaction spreads out laterally and involves multiple atoms of the electrode in the first two atomic layers from the surface.
The range of Eads shows that the nature of adsorption for both H2O and H2S is weak chemisorption/physisorption. The adsorption angle is related to the relative strengths of the Cu–χ, Cu–Hχ and electrostatic interactions. The electrostatic interaction forces the orientation of the adsorbates to be in a vertical hydrogen-up orientation,9,10,36i.e., α = 90°. The adsorption angles of 14.00° and 19.89°, both very far from the 90° imposed by the electrostatic interaction, show that the electrostatic interaction is not the dominant type of interaction for both H2O and H2S. Instead, the covalent interaction is the strongest component of the overall interaction between the adsorbate and the electrode.
The relevant contributions of Cu–χ and Cu–Hχ interactions can be investigated by the projected density of states (PDOS) analysis, which projects the density of states onto individual atoms (or orbitals of an atom). By projecting the DOS onto individual atoms, the contribution of each atom to the adsorption can be studied by comparing the PDOS of the molecules in isolated and adsorbed states. More changes in the PDOS (i.e., shifting and broadening) mean a greater contribution of the corresponding state, or orbital, to the adsorption process. The PDOS plots of H2O/Cu(111) and H2S/Cu(111) are shown in Fig. 2. To display the impact of adsorption, the PDOS of the adsorbates at the isolated (left) and adsorbed (right) states are shown next to each other on the same scale. The energy is measured with respect to the vacuum level, Evac. The dashed horizontal line represents the Fermi level and the shaded area represents the sum of the PDOS of the 3d orbitals of the surface Cu atoms. The cell size of 30 Å × 30 Å × 30 Å was used to compute the PDOS of the isolated molecules and Evac was measured in the direction perpendicular to the direction of the dipole moment to eliminate its impact.
 | ||
| Fig. 2 PDOS of (a) H2O and (b) H2S in isolated (left) and adsorbed (right) settings. A different scale is used for the PDOS of 3d orbitals to increase the visibility of all of the PDOS. | ||
For both H2O and H2S, the highest occupied molecular orbital (HOMO) is composed only of orbitals of χ atoms, commonly referred to as the lone-pair orbital. It can be seen from Fig. 2 that the lone-pair orbitals display the most significant interaction with the 3d orbitals of the surface Cu atoms and also the most change (e.g., peak PDOS value, energy shifting and broadening) upon adsorption compared to other states at lower energies. This reveals that the Cu–χ interaction is the strongest interaction for both H2O and H2S. The stronger Cu–χ interaction is in agreement with the prediction that a small α value corresponds to a strong interaction between χ and the electrode.8–10,38 Additionally, the change in the PDOS of the lone-pair orbitals is slightly greater for H2S, suggesting that the Cu–χ interaction is stronger for H2S than for H2O. Based on the fact that the Cu–χ interaction is the most dominant type of interaction for adsorption, the stronger Eads and larger dCu–χ of H2S compared to that of H2O can be explained by differences between the 2p orbital of O and the 3p orbital of S atoms. The 3p orbital spreads out further in space compared to the 2p orbital with a peak of the electron probability distribution radially farther away from its nucleus. This allows more overlap between the 3p and Cu(111) surface orbitals. Consequently, lone-pair orbitals of H2S mix more with the 3d orbital, which leads to a stronger adsorption at a larger equilibrium distance.
The states below the HOMO are formed by orbitals of both H and χ atoms, and the changes in these states can be linked to the Cu–Hχ interaction. A comparison of the changes in the PDOS of the energy state below the HOMO shows that the Cu–Hχ interaction is stronger for H2S than for H2O. The stronger Cu–S and Cu–HS interactions compared to Cu–O and Cu–HO interactions mean that H2S adsorbs more strongly onto the Cu(111) surface than H2O does, which is consistent with the higher Eads of H2S (Eads = −0.25 eV for H2S and Eads = −0.19 eV for H2O).
Another means of investigating the nature of the interaction is to perform a difference charge density, Δρ, analysis. The Δρ plot is shown in Fig. 3. Δρ can be found by subtracting the charge densities of the clean electrode and the isolated adsorbate from the charge density of the adsorbate–electrode interface, i.e., Δρ = ρads/Cu(111) − ρads − ρCu(111). The Δρ analysis allows one to understand the charge transfer (both the extent of transfer and the participating atoms) when the adsorbate and electrode are interacting. In Fig. 3, the red isosurface represents accumulation of charge and the blue isosurface represents depletion of charge. The figure shows that most of the charge transfer occurs between the adsorbate and the region between the adsorbate and the surface layer, similar to the results found for a water bilayer on Pt(111)39 and Ru(0001).40 Therefore, the term “charge rearrangement” is more appropriate than the term “charge transfer” between the adsorbate and the electrode. A key feature of the charge rearrangement is the depletion of charge in the region between the adsorbate and the Cu atom underneath it (Cu1) and an accumulation of charge around the six surrounding surface Cu atoms which form a hexagonal ring (Cu6). This observation implies an interesting feature of adsorption of H2χ onto Cu(111) – the adsorption of H2χ affects Cu1 and Cu6 in an opposite manner. A further analysis and discussion will follow on this observation.
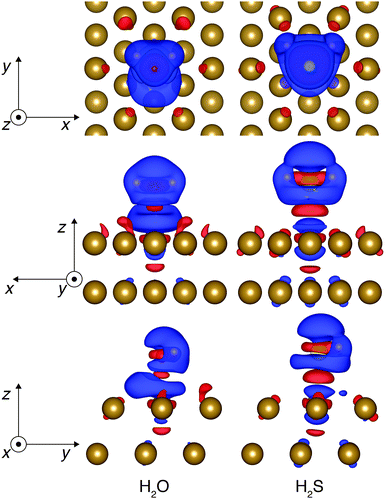 | ||
| Fig. 3 Charge density difference of H2O/Cu(111) (left) and H2S/Cu(111) (right). A blue (red) isosurface represents depletion (accumulation) of charge. | ||
To validate the observations based on Fig. 3, the structural changes in the surface Cu atoms are also analyzed. Table 4 shows the translations of the Cu atom below the adsorbate (dCu1) and the six Cu atoms forming a hexagonal ring (dCu6) around the adsorbate. The direction of dCu1 is almost entirely upwards towards the adsorbate, while the direction of dCu6 is away from the adsorbates (i.e., laterally outwards and vertically downwards). A maximum (dCu6-max), a minimum (dCu6-min) and an average value (dCu6-avg) of the translations of the six Cu atoms are reported for dCu6. The directions of translation would suggest that there is an attractive force between H2χ and Cu1 and a repulsive force between H2χ and Cu6. The greater extent of the translation in the case of H2S adsorption compared to H2O adsorption agrees with the stronger adsorption of H2S indicated by the PDOS analysis and Eads.
| Adsorbate | d Cu1 (Å) | d Cu6-avg (Å) | d Cu6-min (Å) | d Cu6-max (Å) |
|---|---|---|---|---|
| H2O | 0.081 | 0.024 | 0.022 | 0.026 |
| H2S | 0.155 | 0.028 | 0.025 | 0.032 |
Although an attractive force exists between the adsorbate and the Cu atom underneath, one cannot determine the source of the translation of the six surface Cu atoms based on the translation and charge redistribution pattern. It could be either due to the repulsive interaction with the adsorbate, or simply due to the upshift of the Cu atom in the middle of the ring. To further investigate the source of this translation, structure optimization computations were performed without the adsorbate while the Cu1 atom was fixed at the upshifted position. The top three layers except for Cu1 were allowed to move freely. The results show that the six Cu atoms translated almost entirely upwards by 0.011 Å (dCu6-avg = 0.011 Å, dCu6-max = 0.012 Å and dCu6-min = 0.010 Å) when dCu1 = 0.081 Å, and by 0.014 Å (dCu6-avg = 0.014 Å, dCu6-max = 0.015 Å and dCu6-min = 0.014 Å) when dCu1 = 0.155 Å. This shows that the upshift of the Cu1 atom brings the six surrounding Cu atoms with it, causing them to translate in the same upwards direction.
An additional set of the structure optimization computations was carried out to validate the presence of the repulsive type interaction. H2χ was adsorbed onto the surface while the Cu1 atom was fixed at its original position and the remaining Cu atoms in the top three layers and H2χ were fully relaxed. This time, the six Cu atoms translated away from the adsorbate by 0.020 Å (dCu6-avg = 0.020 Å, dCu6-max = 0.022 Å and dCu6-min = 0.018 Å) when H2O was adsorbed, and by 0.041 Å (dCu6-avg = 0.041 Å, dCu6-max = 0.047 Å and dCu6-min = 0.034 Å) when H2S was adsorbed. Therefore, it can be seen that the interaction between H2χ and Cu6 shows a repulsive characteristic. However, it is important to stress that one cannot be sure if the repulsive characteristic is a result of a direct repulsive force between H2χ and Cu6. It is also possible that the presence of H2χ causes the charge to be accumulated around the six Cu atoms (a red isosurface in Fig. 3), which causes the six Cu atoms to repel each other. In either case, the presence of H2χ causes the six Cu atoms to move away from the adsorbate and it is not due to the upshift of the Cu atom in the middle of the ring.
One way to explain the nature of the observed attractive/repulsive pattern is by using a “d-band model”,41,42 which describes the interaction between an adsorbate and a transition metal surface. According to the d-band model, the adsorption energy is greater if a greater extent of the d-band of the metal is filled (i.e., a larger fraction of the d-band is below the Fermi level). This correlation is related to the mobility of d-electrons which move away from the adsorption site to reduce the Pauli repulsion – d-electrons move more (less) freely to reduce the Pauli repulsion if the d-band is less (more) populated. For the case of copper, a nearly fully occupied d-band (d10) makes the d-electrons immobile, which makes Cu(111) hydrophobic. This also agrees well with the weak adsorption energies of H2χ. However, much more mobile sp-electrons move away from the adsorption site to reduce the Pauli repulsion. Using this concept, Schiros et al.43 explained the different water wetting behaviour of Cu(110) compared to Cu(111). They found that although the electronic structure (filled d-band) is the same for Cu(110) and Cu(111), Cu(110) is hydrophilic because it is geometrically corrugated and the sp-electrons preferentially occupy the region between Cu atoms,43 which leaves the adsorption site electron deficient.
A possible scenario that describes the adsorption process which causes the rippled surface pattern is deduced based on the d-model and the mobile sp-electrons of copper. When H2χ is adsorbed, the mobile sp-electrons of the Cu1 atom move out laterally along the surface to Cu6 atoms to minimize the Pauli repulsion force. This facilitates the adsorption of H2χ by making the Cu1 atom electron deficient which helps it to share the electrons with H2χ. The sharing of the electron (i.e., dative bond) involves an attraction force and thus, H2χ pulls the Cu1 atom upwards. The Cu6 atoms with the excess sp-electrons (which will preferentially move towards the surface of Cu or a space between Cu1 and Cu6) repel each other and thus, move away from H2χ. This description matches well with the observed pattern and is also consistent with the conventional understanding that the adsorbed water (and hydrogen sulfide for this work) acts as an electron donor and the electron deficient atop site acts as an electron acceptor.44 The main difference from the conventional picture is that the excess electrons from H2χ are not localized to the adsorption site, but are spread across Cu6.
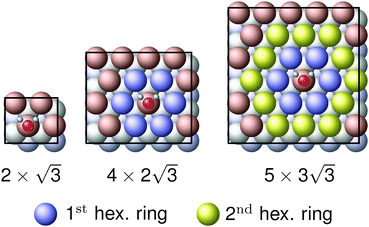
The observed charge redistribution and structural changes in the surface Cu atoms indicate that the adsorbate–electrode interaction spreads out laterally on the surface. This lateral interaction sets a lower bound on the surface size and thus, the supercell of different surface sizes is compared with varying levels of structural relaxations. The surface sizes compared are shown in Fig. 4. The surface sizes of 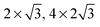 and
and 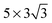 correspond to a 1/4, 1/16 and 1/30 monolayer surface coverage, respectively. The surface coverage is measured in terms of the ratio between the number of adsorbates and the number of surface atoms (e.g., 1 monolayer corresponds to the presence of one adsorbate – H2O or H2S – for every surface Cu atom). It is emphasized that a significant artificial interaction with the periodic images is expected for a
correspond to a 1/4, 1/16 and 1/30 monolayer surface coverage, respectively. The surface coverage is measured in terms of the ratio between the number of adsorbates and the number of surface atoms (e.g., 1 monolayer corresponds to the presence of one adsorbate – H2O or H2S – for every surface Cu atom). It is emphasized that a significant artificial interaction with the periodic images is expected for a 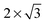 supercell and is included for comparison. Each of the surface sizes is computed with a water monomer adsorbed onto the atop site while 0, 1, 2 and 3 top layers of the electrode are relaxed. For
supercell and is included for comparison. Each of the surface sizes is computed with a water monomer adsorbed onto the atop site while 0, 1, 2 and 3 top layers of the electrode are relaxed. For 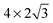 and
and 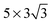 supercells, the results obtained when only the underlying Cu atom is relaxed (Cu1) and when the underlying Cu atom and six surrounding Cu atoms are relaxed (Cu7) are also compared. A comparison of the computation results is shown in Table 5. The H2O/Cu(111) interface is discussed to demonstrate the impact of the surface size of the supercell and the number of relaxed atoms/layers. The same trend is expected for H2S/Cu(111) as it shares similar interaction characteristics (i.e., charge redistribution and structural changes).
supercells, the results obtained when only the underlying Cu atom is relaxed (Cu1) and when the underlying Cu atom and six surrounding Cu atoms are relaxed (Cu7) are also compared. A comparison of the computation results is shown in Table 5. The H2O/Cu(111) interface is discussed to demonstrate the impact of the surface size of the supercell and the number of relaxed atoms/layers. The same trend is expected for H2S/Cu(111) as it shares similar interaction characteristics (i.e., charge redistribution and structural changes).
The comparison of the three surface sizes shows that both the adsorption energy and geometry obtained using a 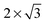 supercell differs significantly from the results obtained using larger cells. This confirms that the lateral size corresponding to the effective surface coverage of 1/4 ML is too small, which allows a significant artificial interaction with its periodic images. The differences between the
supercell differs significantly from the results obtained using larger cells. This confirms that the lateral size corresponding to the effective surface coverage of 1/4 ML is too small, which allows a significant artificial interaction with its periodic images. The differences between the 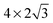 and
and 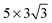 supercells show that the adsorption geometry is more sensitive to the lateral size change than Eads, which agrees with the previous DFT studies on 4d metal surfaces.36,37 Additionally, the greater α and smaller dCu–O pattern for a larger surface supercell shows that the O atom gets closer to the surface with an increased surface size while H atoms remain relatively fixed in height. The increased adsorption strength with a greater level of relaxation and lateral size indicates that the structural changes in the copper electrode facilitates the adsorption of H2χ.
supercells show that the adsorption geometry is more sensitive to the lateral size change than Eads, which agrees with the previous DFT studies on 4d metal surfaces.36,37 Additionally, the greater α and smaller dCu–O pattern for a larger surface supercell shows that the O atom gets closer to the surface with an increased surface size while H atoms remain relatively fixed in height. The increased adsorption strength with a greater level of relaxation and lateral size indicates that the structural changes in the copper electrode facilitates the adsorption of H2χ.
Surface sizes of both 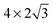 and
and 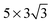 supercells include the hexagonal ring formed by six Cu atoms. However, the latter includes the secondary hexagonal ring which surrounds the first hexagonal ring as shown in Fig. 4. This additional ring acts as a “cushion” where it reduces the interaction of the six Cu atoms in the first ring with the periodic images of H2O. Although the impact of inclusion of the second hexagonal ring is not as severe as the inclusion of the first ring, both adsorption geometry and adsorption energy show changes, following the same pattern when the first ring is included. It is believed that a further reduction in the lateral size from the
supercells include the hexagonal ring formed by six Cu atoms. However, the latter includes the secondary hexagonal ring which surrounds the first hexagonal ring as shown in Fig. 4. This additional ring acts as a “cushion” where it reduces the interaction of the six Cu atoms in the first ring with the periodic images of H2O. Although the impact of inclusion of the second hexagonal ring is not as severe as the inclusion of the first ring, both adsorption geometry and adsorption energy show changes, following the same pattern when the first ring is included. It is believed that a further reduction in the lateral size from the 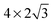 supercell will lead to the computation results that deviate more from that obtained using the
supercell will lead to the computation results that deviate more from that obtained using the 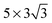 supercell. This is also indicated by a large variation in adsorption geometry and energy in the comparison shown in Table 3, even for the cases where a first hexagonal ring is included (Θ = 1/9). The impact of the relaxed layers is also compared. The contribution of the level of relaxation is significant up to the top two layers, but a further relaxation does not alter the computation results considerably.
supercell. This is also indicated by a large variation in adsorption geometry and energy in the comparison shown in Table 3, even for the cases where a first hexagonal ring is included (Θ = 1/9). The impact of the relaxed layers is also compared. The contribution of the level of relaxation is significant up to the top two layers, but a further relaxation does not alter the computation results considerably.
It should be noted that the lower bound of the surface supercell size and the number of relaxed layers vary from one type of metal to another. The vertical translations of the atom underneath the adsorbed H2O are reported for several types of metal surfaces such as Ru(0001), Rh(111), Pd(111), Pt(111), Cu(111), Ag(111) and Au(111) by Michaelides et al.10 The comparison was made using a 2 × 2 supercell and thus, the direction and magnitude of the translation of the surrounding Cu atoms are missing. However, the comparison showed that Cu(111) displays the largest structural changes among the types of surfaces they investigated. This suggests that Cu(111) is structurally more sensitive to adsorption than the metal surfaces considered in ref. 10, which means that other types of metal surfaces may be less influenced by the supercell size. Lastly, it can be seen from Table 3 that the rippled structure of Cu(111) increases the adsorption energy by around 0.02 eV. Although its contribution is small, the presence of the water molecules in the neighboring adsorption sites (e.g., a water bilayer structure which takes a hexagonal arrangement35) interrupts the rippled pattern which may reduce the adsorption strength of the water molecule. This may contribute to the hydrophobic nature of the Cu(111) surface where water does not form a bilayer structure but instead forms 3D clusters.2,45,46 However, further investigation is needed to determine the impact of the rippled surface on the wettability of the surface.
The reported results are based on Cu(111), but a similar type of interaction is expected for other transition metals to a varying extent. The fundamental understanding of the nature of adsorption interaction and types of forces that exist is becoming increasingly more important, especially for the cases where multiple adsorbates are present and interact with another. For example, a repulsive force between the adsorbates has been recognized and recently been utilized to engineer a pattern of adsorbates on the surface or to control the extent of charge transfer between the adsorbate and the surface (e.g., free-base porphine on Ag(111),47 benzene and its derivatives on Cu(111),48etc.). Recently, the adsorbate–adsorbate interaction was taken into account for studying the electrocatalytic CO2 reduction and it was shown to affect the catalytic activity.49 In addition, the structural changes in the electrode surface, to be discussed in detail in the next section, is correlated to the translation of the adsorbates when an external electric field is applied to the interface. The rotation and translation of the adsorbate are of particular interest in energy storage and catalysis applications because it is directly linked to the electronic polarizability of the interface.
3.2 Impact of an external electric field
The characteristics of the adsorption of H2O and H2S onto Cu(111) such as the adsorption energy and angle, the relative strengths of the Cu–χ interaction, the surface deformation and charge redistribution have been discussed in the absence of the external electric field. These characteristics can be used to predict the response of the interfaces when they are exposed to an external electric field. This section discusses the computation results in the presence of the external electric field and which information from the previous analysis (i.e., in the absence of the electric field) is relevant in predicting the response of the interface to the electric field.A normal external electric field is applied to both H2O/Cu(111) and H2S/Cu(111) up to ±1 × 1010 V m−1 with a ±1 × 109 V m−1 increment. The sign of the electric field is positive when the direction of the field is directed outwards from the surface, which corresponds to the direction of the electric field when the surface is positively charged. The adsorption angle, α, of both adsorbates is shown with respect to the external electric field in Fig. 5a. The orientation of both H2O and H2S becomes more hydrogen-up and hydrogen-down with the positive and negative electric fields, respectively. It can also be seen that the rotation of H2O is more sensitive to the external electric field than H2S, which remains rigid without much rotation. The rotation of H2O also shows that it is asymmetric with respect to the direction of the electric field – it is more sensitive to a negative electric field. The asymmetry and greater rotation for a negative electric field agrees with the previously observed pattern for a water molecule adsorbed onto gold9 and copper32 electrodes.
The difference in the rotation of the two adsorbates can be explained/predicted by two parameters that are closely linked to the degree to which the adsorbates respond to the external electric field. The first parameter is the adsorption energy, Eads, which is the energy that binds the adsorbate to the electrode. A higher Eads means that the adsorbate is bound strongly to the surface via covalent interactions between the adsorbate and Cu atoms of the electrode. The relative contribution of the Cu–χ and Cu–Hχ interactions to Eads changes as the orientation of the adsorbate is varied8 and thus, Eads is used as a parameter to describe the adsorbate–electrode interaction. As discussed above, a significant contribution of the overall adsorbate–electrode interaction comes from the Cu atoms that are not directly underneath the adsorbate. The interaction with multiple Cu atoms indicates that the higher Eads is linked to how strongly the adsorbate is “locked” in the original orientation.
The second parameter is the dipole moment of the adsorbate. In the absence of an external field, the covalent interaction dominates over the electrostatic interaction, which is a dipole–dipole interaction between the adsorbate and its image in the electrode. When the external electric field is applied, a dipole–field interaction is introduced and its strength increases with the higher electric field. While the electric field is the control parameter, the dipole moment is the characteristic of the molecule itself and remains relatively constant. Therefore, it is expected that a higher Eads and a lower dipole moment of an adsorbate lead to an electrode/adsorbate interface that is less sensitive to the external field and vice versa.
H2S has a higher Eads and a lower dipole moment compared to H2O, both of which make H2S less responsive to the external electric field compared to H2O. In other words, H2S requires a higher electric field compared to H2O in order to make a complete rotation (α = ±90°). This description matches well with the large difference in Δα in Fig. 5a. The inertness of H2S is not only observed in the small change in α, but also in the change in the bond angle of the adsorbate as shown in Fig. 5b – the range of observed bond angle for H2O is around 4° while the bond angle of H2S remains virtually unchanged. The asymmetry and higher sensitivity to a negative electric field is again observed for the bond angle change for H2O. The electrostatic energy due to the external electric field is determined by computing the difference in Eads with and without the field while all of the atoms are fixed at their relaxed locations (in the presence of the field). The difference in Eads corresponds to the electrostatic energy due to the field. The electrostatic energy due to E = +1 × 109 V m−1 is 13.3% and 8.7% of Eads for H2O and H2S, respectively, indicating that the field alters the adsorption characteristics of H2O more significantly.
The rotations of H2O and H2S can also be explained using the electrostatic model of Maschhoff and Cowin.50 According to the model, the total energy acting to rotate the adsorbate from a flat to a complete hydrogen-up orientation is written as U = − ½ μ0E. U, μ0 and E are the total energy of the dipole, dipole moment of the adsorbate and magnitude of the external electric field, respectively. At high electric field values, the polarization energy and dipole image energy can be neglected for simplicity. Using the expression above, the electrostatic energy acting to rotate H2O and H2S from a flat to a complete hydrogen-up position is −0.19 eV and −0.10 eV at E = +1 × 1010 V m−1, respectively. This energy is about the same as the adsorption energy of H2O and thus can rotate water to a hydrogen-up orientation, which is consistent with our observation. On the other hand, the electrostatic energy of −0.10 eV for H2S at E = +1 × 1010 V m−1 is far smaller than its adsorption energy of −0.25 eV, which agrees with the predicted small rotation.
The vertical translations of H2O and H2S are shown in Fig. 5c, where the centres of mass in the absence of the external electric field are used as reference points to measure the translation. A positive and negative translation corresponds to the adsorbate moving away and towards the electrode, respectively. Similar to the rotation, H2S remains less sensitive to the electric field than H2O and the adsorbates translate more in response to a negative field compared to the positive electric field. Except for few small positive electric field values where the translation of H2S is slightly downwards, the translation is upwards for both negative and positive electric fields. This pattern contrasts with what is reported for H2O/Au9 where the translation is towards the surface for positive electric fields while it is away from the surface for negative electric fields. Another difference from the H2O/Au case is that the magnitude of the translation for H2O/Cu is almost twice for a negative field and half for a positive electric field compared to H2O/Au. These differences, both in direction and magnitude, are related to the structural changes in the copper surface, which are greater for copper compared to gold.
The vertical displacements of Cu1 and Cu6 with respect to the external electric field are shown in Fig. 5d, where the displacement is measured with respect to their original positions prior to the adsorption. The average value of the six Cu atoms is used for measuring the displacement of Cu6. Similar to the structural changes in the adsorbates, the extent of the surface deformation also changes as the external field is applied. It can be seen that (i) there is a significant vertical translation of Cu1 and the direction of the translation of Cu1 is downward for a negative electric field and upward for a positive electric field.
The translation pattern of the adsorbates and the underlying Cu atoms (Fig. 5c and d) reveals some interesting features of the interface under the influence of an external electric field. Firstly, when the negative electric field is applied, the adsorbates not only rotate to become more hydrogen-down oriented, but they also move away from the electrode. The vertical translations of both adsorbate and the underlying Cu atom can be explained by the fact that the rotation of the adsorbates reduces the originally dominant Cu–χ interaction as O and S atoms face away from the interacting Cu electrode. As a consequence, the attraction force between Cu1 and the adsorbate becomes weaker, causing the adsorbates to move away from the surface while Cu1 approaches its original position in the absence of the adsorbates. The weaker interaction between H2O and Cu(111) at strong negative electric fields coincides with the more mobile H2O molecule on Cu(111) when the electrode is negatively charged as suggested by Zhang et al.32 However, the change in the binding strength is expected to be much less for H2S/Cu(111) at negative electric fields.
An opposite trend is shown when a positive electric field is applied – the rotation makes O and S atoms face toward the surface. Although the positive vertical translation of the adsorbates is observed for most of the points, it can be seen from Fig. 5d that the vertical translation of the underlying Cu atom is greater in magnitude. This shows that the distance between the adsorbate and the underlying Cu atom decreases as the magnitude of the positive electric field increases. It is difficult to determine definitively whether the adsorbates move towards or away from the surface when the positive electric field is applied because the surface becomes further deformed from its original flat structure. However, the decreased distance between H2χ and Cu1 suggests that the binding strength between the adsorbates and Cu1 is greater when a positive electric field is applied. No considerable change is observed for H2S/Cu(111), except that the Cu(111) surface is further deformed.
The translation of the adsorbates for H2O/Cu(111) and H2S/Cu(111) differs significantly from the case of H2O/Au both in magnitude and direction. The difference is related to the structural sensitivity of the electrode surfaces to the presence of the adsorbates and the electric field. For H2O/Au, the water molecule is mainly responsible for the geometric response of the interface to the electric field where H2O moves towards and away from the surface when the positive and negative electric fields are applied, respectively. On the other hand, the structural changes in the Cu surface contribute significantly to the translation of the H2O/Cu(111) interface. The Cu surface plays an even larger role in the response of the H2S/Cu(111) interface, where the translation of the Cu1 atom is comparable to the translation of the H2S molecule. Therefore, the surface structure of the electrode needs to be included in the analysis of the H2χ/Cu(111) interface both in the absence and presence of the electric field, unlike most of the reported studies of metal/adsorbate systems.
4 Conclusions
The investigation suggests that the interaction between the lone pair orbitals of the adsorbates and the electrode is the largest contributor to the overall adsorption interaction although the role of Cu–Hχ interaction cannot be neglected. The Δρ profile and the structural changes show that a considerable part of the overall adsorbate–electrode interaction is attributed to the interaction with the six surrounding surface Cu atoms that form a hexagonal ring structure. The adsorption of H2χ attracts the underlying Cu atom towards the adsorbate while the six surrounding Cu atoms move away from it. The presence of the mixed attractive and repulsive behaviour of the adsorbates could have a major implication in understanding the adsorbate–adsorbate interaction, which is becoming increasingly more important. The interaction between H2χ and the electrode penetrates two layers below the electrode surface and spreads out laterally. This requires a large lateral size for the supercell to accurately describe the H2χ/Cu(111) interaction.When an external electric field is applied, the dipole–field interaction increases the electrostatic energy and the molecule starts to rotate to become more hydrogen-up oriented for positive electric fields and hydrogen-down oriented for negative electric fields. The computation results show that a higher adsorption energy and a lower dipole moment lead to an interface that responds more sensitively to the external electric field. However, it is found that for the case of Cu(111), the structural changes in the electrode surface need to be considered in order to understand the translation behaviour of the adsorbate. Therefore, the adsorption energy, the dipole moment of the adsorbates and the structural sensitivity of the electrode surface (i.e., the extent of deformation of the surface structure upon the adsorption of molecules and the presence of the electric field) are the main parameters that are linked to how the interface reacts to the electric field. This relationship may serve as a stepping-stone towards developing a complete framework for the interaction of H2O and similar molecules on transition metal electrodes in the presence and absence of an external electric field. The applicability of the observed trends to interfaces consisting of other transition metals and water-like molecules requires further investigations.
The most prominent difference in the behaviour of H2O and H2S on Cu(111) is in their response to an electric field. H2S remains rigid with a very small rotation and translation for a range of normal external electric fields up to ±1 × 1010 V m−1. The structural changes in the electrode surface are comparable to the geometric changes in H2S. On the other hand, H2O undergoes a near complete rotation and significant translation. This means that the relative behaviour of H2O/Cu(111) and H2S/Cu(111) such as the binding energy and mobility of the adsorbates along the surface can be easily tuned by simply charging the Cu electrode. The findings of this work can be applied to systems where H2O and H2S coexist, such as a water gas shift reaction on Cu(111).6
Acknowledgements
The authors thank the support from the NSERC CREATE Program. Computations were performed on the gpc supercomputer at the SciNet HPC Consortium. SciNet is funded by the Canada Foundation for Innovation under the auspices of Compute Canada; the Government of Ontario; Ontario Research Fund – Research Excellence; and the University of Toronto.51References
- E. M. Stuve, Chem. Phys. Lett., 2012, 519–520, 1–17 CrossRef CAS.
- J. Carrasco, A. Hodgson and A. Michaelides, Nat. Mater., 2012, 11, 667–674 CrossRef CAS PubMed.
- J. Rossmeisl, E. Skúlason, M. E. Bjorketun, V. Tripković and J. K. Nørskov, Chem. Phys. Lett., 2008, 466, 68–71 CrossRef CAS.
- A. A. Phatak, W. N. Delgass, F. H. Ribeiro and W. F. Schneider, J. Phys. Chem. C, 2009, 113, 7269–7276 CAS.
- J. Ren and S. Meng, J. Electrochem. Soc., 2008, 77, 054110 Search PubMed.
- C. Callaghan, I. Fishtik, R. Datta, M. Carpenter, M. Chmielewski and A. Lugo, Surf. Sci., 2003, 541, 21–30 CrossRef CAS.
- Y. Santiago-Rodrguez, J. A. Herron, M. C. Curet-Arana and M. Mavrikakis, Surf. Sci., 2014, 627, 57–69 CrossRef.
- A. Michaelides, Appl. Phys. A: Mater. Sci. Process., 2006, 85, 415–425 CrossRef CAS.
- A. Huzayyin, J. H. Chang, K. Lian and F. Dawson, J. Phys. Chem. C, 2014, 118, 3459–3470 CAS.
- A. Michaelides, V. Ranea, P. de Andres and D. King, Phys. Rev. Lett., 2003, 90, 216102 CrossRef CAS PubMed.
- P. N. Abufager, P. G. Lustemberg, C. Crespos and H. F. Busnengo, Langmuir, 2008, 24, 14022–14026 CrossRef CAS PubMed.
- W. S. Benedict, N. Gailar and E. K. Plyler, J. Chem. Phys., 1956, 24, 1139–1165 CrossRef CAS.
- H. C. Allen, Jr and E. K. Plyler, J. Chem. Phys., 1956, 25, 1132–1136 CrossRef.
- R. D. Nelson, Jr, D. R. Lide, Jr and A. A. Maryott, Selected Values of Electric Dipole Moments for Molecules in the Gas Phase, Natl. Stand. Ref. Data Ser. (U. S., Natl. Bur. Stand.), U.S. Government Printing Office, Washington, DC, 1967 Search PubMed.
- J. L. C. Fajn, F. Illas and J. R. B. Gomes, J. Chem. Phys., 2009, 130, 224702 CrossRef PubMed.
- J. A. Rodriguez, J. C. Hanson, D. Stacchiola and S. D. Senanayake, Phys. Chem. Chem. Phys., 2013, 15, 12004 RSC.
- Q.-L. Tang, S.-R. Zhang and Y.-P. Liang, J. Phys. Chem. C, 2012, 116, 20321–20331 CAS.
- T. T. M. Tran, C. Fiaud and E. M. M. Sutter, Corros. Sci., 2005, 47, 1724–1737 CrossRef CAS.
- P. Giannozzi, S. Baroni, N. Bonini, M. Calandra, R. Car, C. Cavazzoni, D. Ceresoli, G. L. Chiarotti, M. Cococcioni, I. Dabo, A. D. Corso, S. de Gironcoli, S. Fabris, G. Fratesi, R. Gebauer, U. Gerstmann, C. Gougoussis, A. Kokalj, M. Lazzeri, L. Martin-Samos, N. Marzari, F. Mauri, R. Mazzarello, S. Paolini, A. Pasquarello, L. Paulatto, C. Sbraccia, S. Scandolo, G. Sclauzero, A. P. Seitsonen, A. Smogunov, P. Umari and R. M. Wentzcovitch, J. Phys.: Condens. Matter, 2009, 21, 395502 CrossRef PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- J. Neugebauer and M. Scheffler, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 16067–16080 CrossRef CAS.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Solid State, 1976, 13, 5188–5192 CrossRef.
- R. Ramprasad, N. Shi and C. Tang, in Dielectric Polymer Nanocomposites, ed. J. K. Nelson, Springer, US, 2010, ch. 5, pp. 133–161 Search PubMed.
- NIST Chemistry WebBook, NIST Standard Reference Database Number 69, ed. P. J. Linstrom and W. G. Mallard, National Institute of Standards and Technology, Gaithersburg MD, 2014, p. 20899 Search PubMed.
- W. Kohn, Rev. Mod. Phys., 1999, 71, 1253–1266 CrossRef CAS.
- N. W. Ashcroft and N. D. Mermin, Solid State Physics, Holt, Rinehart and Winston, New York, 1st edn, 1976 Search PubMed.
- H. B. Michaelson, J. Appl. Phys., 1977, 48, 4729–4733 CrossRef CAS.
- M. P. Hyman, B. T. Loveless and J. W. Medlin, Surf. Sci., 2007, 601, 5382–5393 CrossRef CAS.
- R. Nadler and J. F. Sanz, J. Mol. Model., 2011, 18, 2433–2442 CrossRef PubMed.
- P. Zhang, W. T. Zheng and Q. Jiang, J. Phys. Chem. C, 2010, 114, 19331–19337 CAS.
- Q.-L. Tang and Z.-X. Chen, Surf. Sci., 2007, 601, 954–964 CrossRef CAS.
- A. Groß, Surf. Sci., 2012, 606, 690–691 CrossRef.
- A. Poissier, S. Ganeshan and M. V. Fernández-Serra, Phys. Chem. Chem. Phys., 2011, 13, 3375 RSC.
- J. Carrasco, A. Michaelides and M. Scheffler, J. Chem. Phys., 2009, 130, 184707 CrossRef PubMed.
- R. Nadler and J. F. Sanz, J. Chem. Phys., 2012, 137, 114709 CrossRef PubMed.
- V. Ranea, A. Michaelides, R. Ramrez, J. Vergés, P. de Andres and D. King, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 69, 205411 CrossRef.
- S. Schnur and A. Groß, New J. Phys., 2009, 11, 125003 CrossRef.
- G. Materzanini, G. Tantardini, P. Lindan and P. Saalfrank, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 155414 CrossRef.
- B. Hammer and J. K. Nørskov, Nature, 1995, 376, 238–240 CrossRef CAS.
- B. Hammer and J. K. Nørskov, Surf. Sci., 1995, 343, 211–220 CrossRef CAS.
- T. Schiros, O. Takahashi, K. J. Andersson, H. Öström, L. G. M. Pettersson, A. Nilsson and H. Ogasawara, J. Chem. Phys., 2010, 132, 094701 CrossRef CAS PubMed.
- P. A. Thiel and T. E. Madey, Surf. Sci. Rep., 1987, 7, 211–385 CrossRef CAS.
- M. Mehlhorn and K. Morgenstern, Phys. Rev. Lett., 2007, 99, 246101 CrossRef PubMed.
- M. Meyer, J. Stähler, D. O. Kusmierek, M. Wolf and U. Bovensiepen, Phys. Chem. Chem. Phys., 2008, 10, 4932–4938 RSC.
- F. Bischoff, K. Seufert, W. Auwärter, S. Joshi, S. Vijayaraghavan, D. Écija, K. Diller, A. C. Papageorgiou, S. Fischer, F. Allegretti, D. A. Duncan, F. Klappenberger, F. Blobner, R. Han and J. V. Barth, ACS Nano, 2013, 7, 3139–3149 CrossRef CAS PubMed.
- S. Simpson and E. Zurek, J. Phys. Chem. C, 2012, 116, 12636–12643 CAS.
- C. Shi, H. A. Hansen, A. C. Lausche and J. K. Nørskov, Phys. Chem. Chem. Phys., 2014, 16, 4720–4727 RSC.
- B. L. Maschhoff and J. P. Cowin, J. Chem. Phys., 1994, 101, 8138 CrossRef CAS.
- C. Loken, D. Gruner, L. Groer, R. Peltier, N. Bunn, M. Craig, T. Henriques, J. Dempsey, C.-H. Yu, J. Chen, L. J. Dursi, J. Chong, S. Northrup, J. Pinto, N. Knecht and R. V. Zon, J. Phys.: Conf. Ser., 2010, 256, 012026 CrossRef.
| This journal is © the Owner Societies 2015 |