Interstellar H adsorption and H2 formation on the crystalline (010) forsterite surface: a B3LYP-D2* periodic study
Abstract
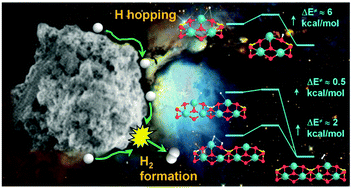
The physisorption/chemisorption of atomic hydrogen on a slab model of the Mg2SiO4 forsterite (010) surface mimicking the interstellar dust particle surface has been modeled using a quantum mechanical approach based on periodic B3LYP-D2* density functional calculations (DFT) combined with flexible polarized Gaussian type basis sets, which allows a balanced description of the hydrogen/surface interactions for both minima and activated complexes. Physisorption of hydrogen is barrierless, very weak and occurs either close to surface oxygen atoms or on Mg surface ions. The contribution of dispersion interactions accounts for almost half of the adsorption energy. Both the hydrogen adsorption energy and barrier to hydrogen jump between equivalent surface sites are overestimated compared to experimental results meant to simulate the interstellar conditions in the laboratory. The hydrogen atom exclusively chemisorbs at the oxygen site of the forsterite (010) surface, forming a SiOH surface group and its spin density being entirely transferred to the neighboring Mg ion. Barrier for chemisorption allows rapid attachment of H at the surface at 100 K, but prevents the same process from occurring at 10 K. From this H-chemisorbed state, the second hydrogen chemisorption mainly occurs on the neighboring Mg ion, thus forming a Mg–H surface group, giving rise to a surface species stabilized by favorable electrostatic interactions between the OH⋯H–Mg pair. The formation of molecular hydrogen at the (010) forsterite surface adopting a Langmuir–Hinshelwood mechanism takes place either starting from two physisorbed H atoms with an almost negligible kinetic barrier through a spin–spin coupling driven reaction or from two chemisorbed H atoms with a barrier surmountable even at T higher than 10 K. We also suggest that a nanosized model of the interstellar dust built from a replica of the forsterite unit cell is able to adsorb half the energy released by the H2 formation by increasing its temperature by about 50 K which could then radiate in about 0.02 s.

 Please wait while we load your content...
Please wait while we load your content...