Exploring the nature of interactions among thiophene, thiophene sulfone, dibenzothiophene, dibenzothiophene sulfone and a pyridinium-based ionic liquid†
Abstract
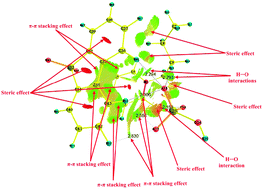
In order to gain an understanding of the nature of the interactions among thiophene (TS), thiophene sulfone (TSO2), dibenzothiophene (DBT), dibenzothiophene sulfone (DBTO2) and the ionic liquid N-butylpyridinium hydrogen sulfate ([BPY][HSO4]), a systematic investigation has been carried out using ab initio methods. The most stable structures indicate that both [BPY]+ and [HSO4]− play crucial roles in the interactions between TS, TSO2, DBT, DBTO2 and [BPY][HSO4]. Analyses of the most stable optimized structures suggest the occurrence of steric effects, π–π stacking effects, hydrogen bonds, and dihydrogen bonds. The π–π stacking effect in [BPY][HSO4]–TSO2/[BPY][HSO4]–DBTO2 is less significant than that in [BPY][HSO4]–TS/[BPY][HSO4]–DBT, as TSO2 and DBTO2 are more nucleophilic than TS and DBT, resulting in stronger interactions between [BPY][HSO4] and TSO2/DBTO2 than between [BPY][HSO4] and TS/DBT. Thermodynamical data also demonstrate that TSO2/DBTO2 are more prone to interact with [BPY][HSO4] compared with TS/DBT.

 Please wait while we load your content...
Please wait while we load your content...