Green synthesis of nanorod Ni(salen) coordination complexes using a simple hydrothermal method
Abstract
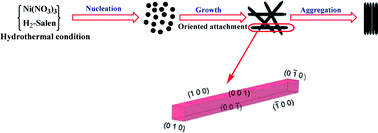
One-dimensional nanorods of a Ni(salen) coordination compound were synthesized by a facile hydrothermal route in green solvent at various times and temperatures without any surfactant or capping agent. The structure and morphology of the as-prepared complexes were investigated by using elemental analysis, 1H NMR, Fourier-transform infrared spectroscopy (FT-IR), UV-vis spectroscopy, X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and thermogravimetric analysis (TGA). 1H NMR, FT-IR and elemental analysis confirmed the formation of a Ni(salen) complex. The SEM and TEM images revealed that the products were nanorods with a diameter of about 40–50 nm. Comparison of the Ni(salen) complexes prepared by hydro/solvothermal method in different solvents (H2O, EtOH and CH3CN) with the Ni(salen) prepared under reflux conditions illustrates that the product of the hydrothermal route has a higher purity. A reasonable growth mechanism for the formation of a Ni(salen) nanorod complex is also proposed.

 Please wait while we load your content...
Please wait while we load your content...