A population balance model for solvent-mediated polymorphic transformation in unseeded solutions
Abstract
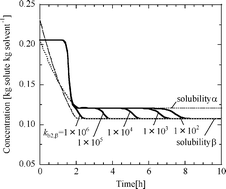
A new population balance model for solvent-mediated polymorphic transformation is presented. The model takes into account the secondary nucleation caused by nuclei grown crystals as well as the primary nucleation. Numerical simulation was performed for crystallization of a hypothetical enantiotropic dimorphic compound in an unseeded solution. In the simulation, particular attention was paid to the effect of secondary nucleation of the stable polymorph on the transformation time. The simulated transformation time decreased with an increase in the secondary nucleation rate of the stable polymorph. Reported experimental data on the effect of stirrer speed were explained by the secondary nucleation-mediated mechanism, in which the secondary nucleation rate is assumed to increase with an increase in stirrer speed. The effect of scale-up on the transformation time was also suggested to be explained by the secondary nucleation-mediated mechanism.

 Please wait while we load your content...
Please wait while we load your content...