Synthesis and luminescence of uniform europium-doped bismuth fluoride and bismuth oxyfluoride particles with different morphologies†
Abstract
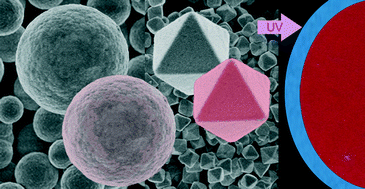
Facile synthesis routes have been developed for the preparation of uniform cubic bismuth fluoride and bismuth oxyfluoride particles. The synthesis methods are based on homogeneous precipitation reactions at 120 °C in solutions of bismuth nitrate and sodium tetrafluoroborate precursors in polyol-based solvents. Both the nature of the solvent and the heating modes (conventional or microwave-assisted heating) have a remarkable effect on the morphology and crystallinity of the resulting particles. Thus, polycrystalline spheres of α-BiF3 with a mean diameter ranging from 1.2 to 2 μm could be obtained by heating solutions with the appropriate reagent concentrations in a mixture of ethylene glycol and glycerol (1 : 1 by volume) using a conventional oven, whereas octahedral single crystals of α-BiOyF3−2y with mean edges ranging from 250 nm to 920 nm precipitated when using a diethylene glycol–water mixture (8 : 2 in volume) as solvent and a microwave reactor for heating. To explain these different morphological and structural features, the mechanism of formation of such particles was investigated. Both kinds of particles were also doped with Eu3+, and both the morphological and luminescence properties of the resulting materials were evaluated. It was found that the luminescence intensity of the europium-doped α-BiOyF3−2y nanoparticles was higher than that of the europium-doped α-BiF3 sub-micrometric spheres, which was associated with the higher crystallinity of the former. Moreover, the presence of oxygen in the europium-doped α-BiOyF3−2y samples permits the excitation of the europium cations through an Eu–O energy transfer process, which results in a much higher luminescence intensity with respect to that corresponding to the direct excitation of the europium cations. Finally, the effect of the amount of dopant on the luminescence properties of the phosphors was also evaluated.

 Please wait while we load your content...
Please wait while we load your content...